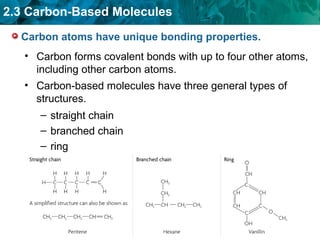
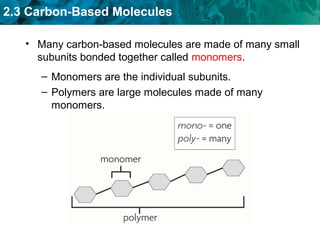
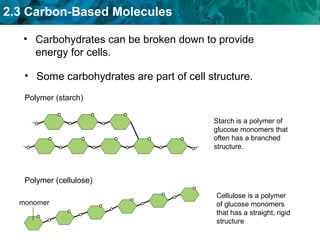
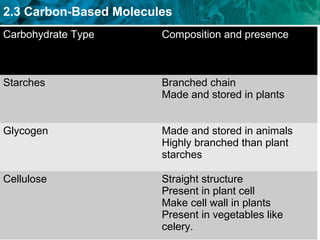
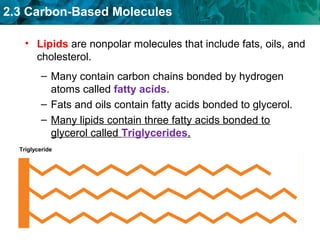
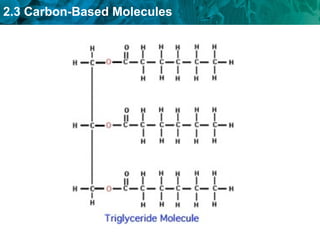
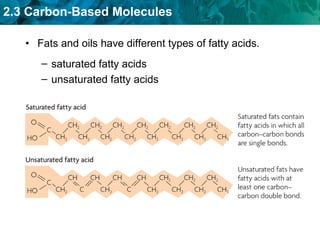
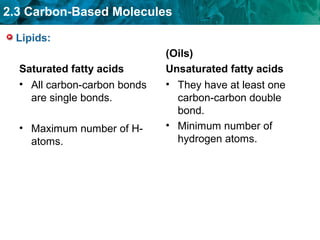
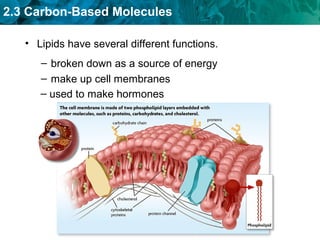
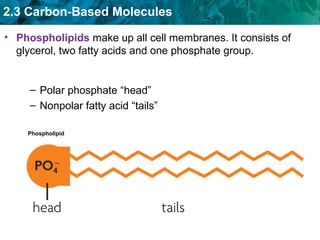
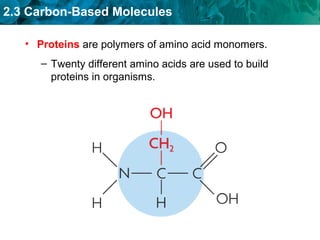
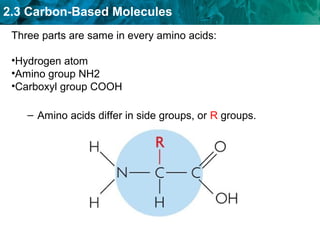
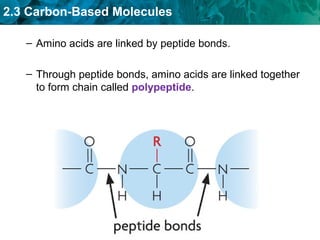
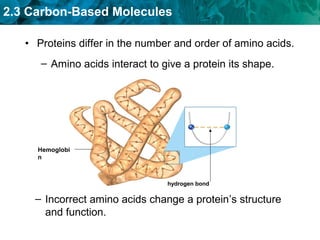
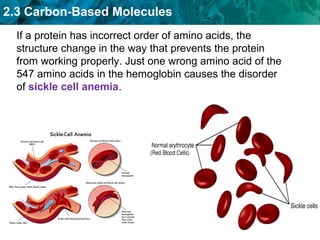
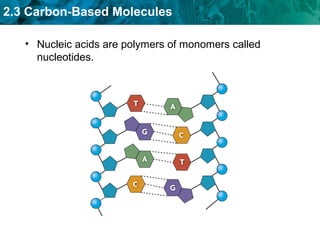
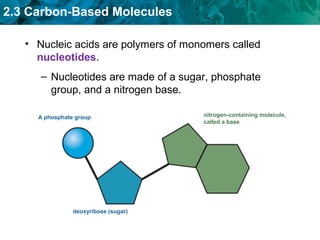
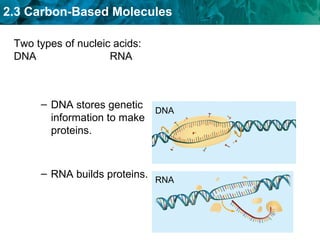
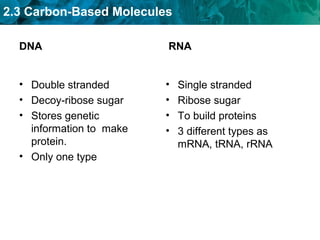
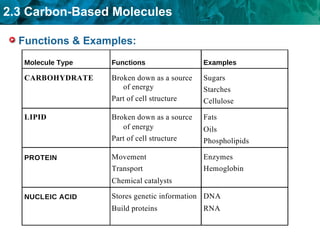
Carbon-based molecules like carbohydrates, lipids, proteins, and nucleic acids are the foundation of life. They have unique structures and functions. Carbohydrates include sugars and starches that can provide energy or be part of cell structure. Lipids include fats, oils, and phospholipids that form cell membranes and can also provide energy. Proteins are made of amino acids linked through peptide bonds and perform important functions like movement and chemical reactions. Nucleic acids like DNA and RNA store genetic information and build proteins.