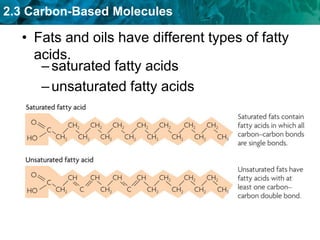
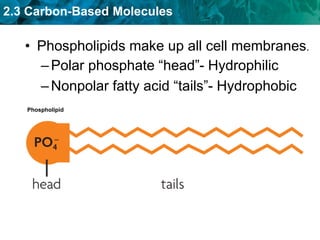
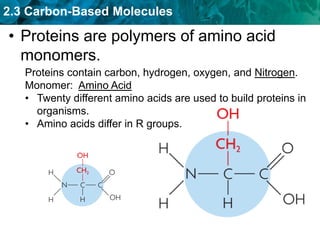
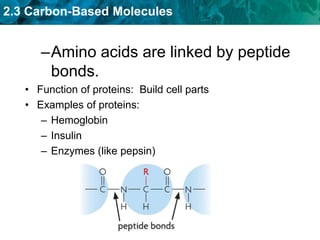
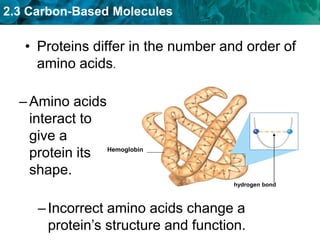
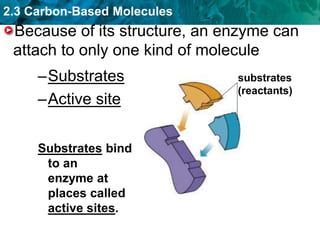
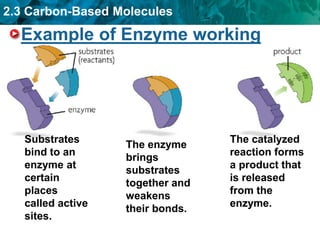
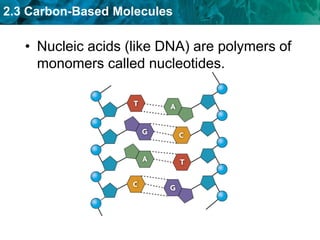
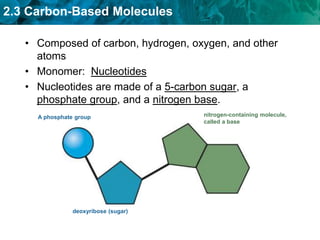
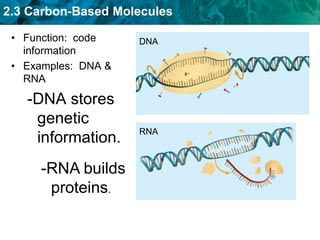
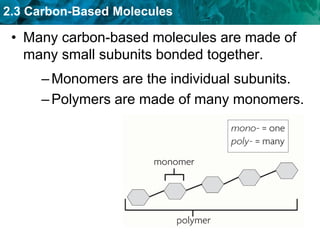
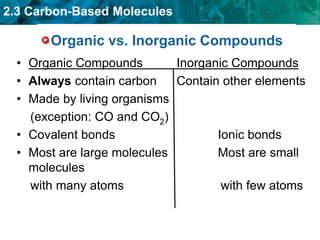
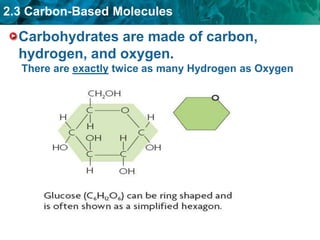
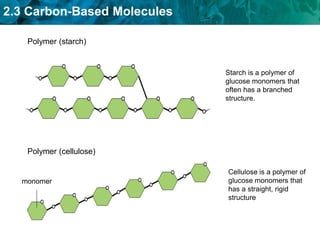
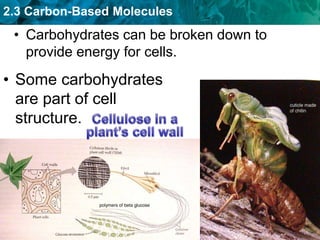
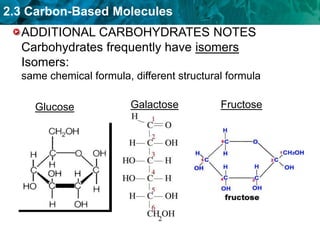
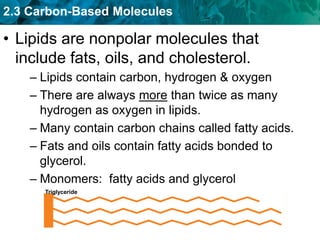
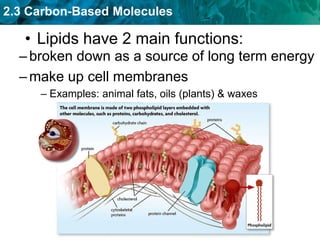
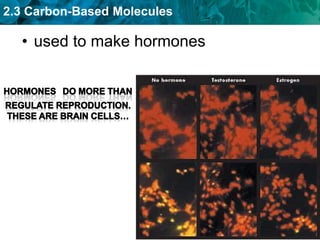
This document discusses carbon-based molecules, which are the foundation of life. It describes the four main types of carbon-based molecules found in living things: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates, lipids, proteins, and nucleic acids are all polymers made of smaller monomer subunits like sugars, fatty acids, amino acids, and nucleotides, respectively. The document provides details on the structures, functions, and examples of each type of carbon-based molecule.




![2.3 Carbon-Based Molecules
Unsaturated Fats
• Generally found in plants
– Examples:
- olive oil, corn oil, sunflower oil, peanut oil
• Liquid at room temperature
• Missing Hydrogen atoms
– [Causes double bonds between C atoms]](https://image.slidesharecdn.com/bio2-230126170002-6c0e68dd/85/bio-2-3-carbon-chemistry-ppt-18-320.jpg)