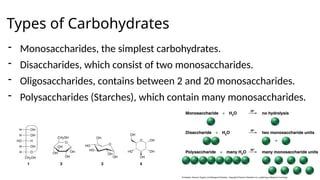
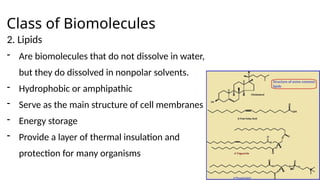
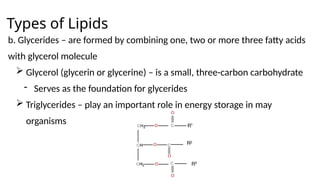
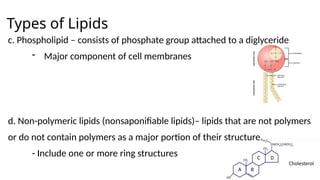
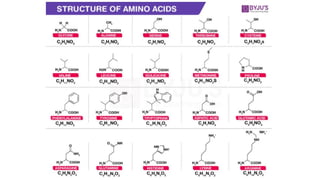
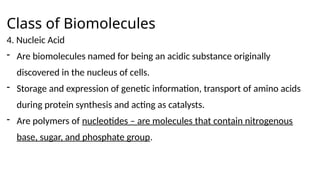
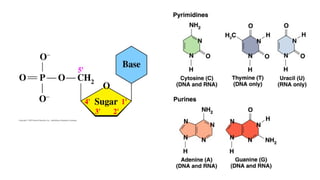
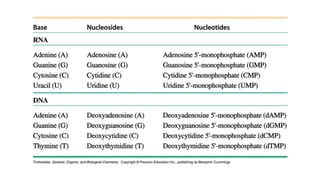
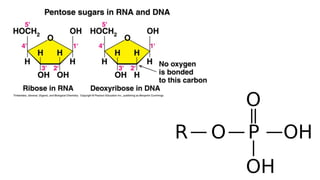
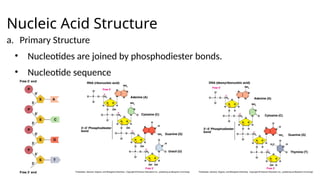
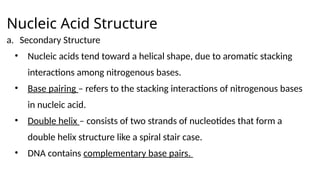
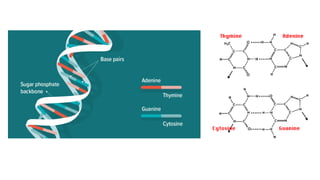
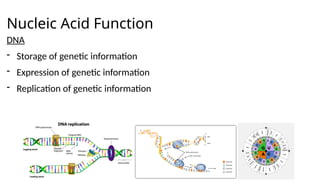
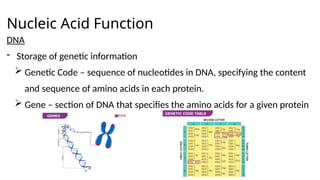
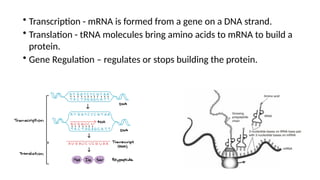
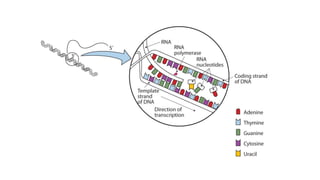
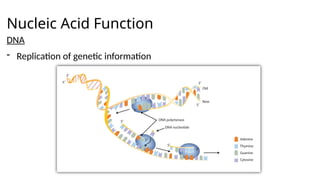
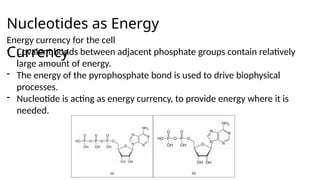
The document provides an overview of biomolecules, summarizing their classifications, structures, and functions. It categorizes biomolecules into carbohydrates, lipids, proteins, and nucleic acids, detailing their properties and roles in living organisms. Key elements like functional groups and biochemical processes such as protein synthesis and energy transfer are also discussed.