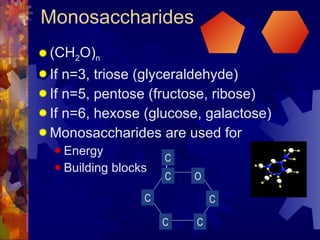
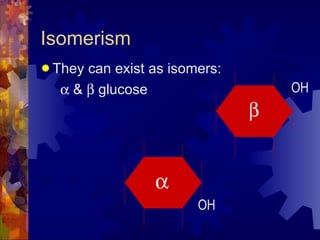
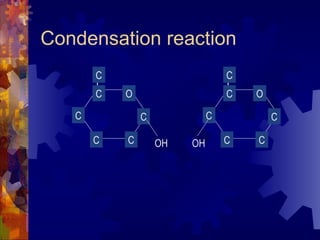
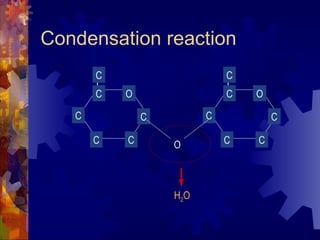
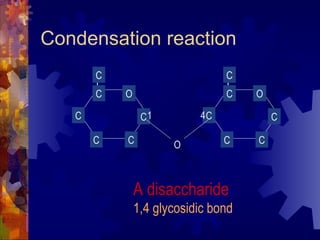
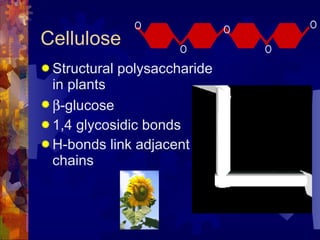
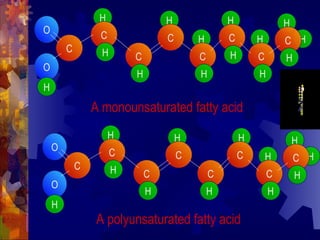
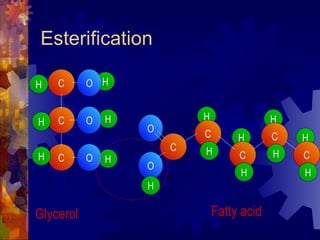
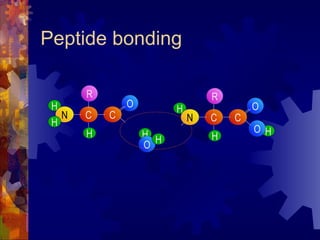
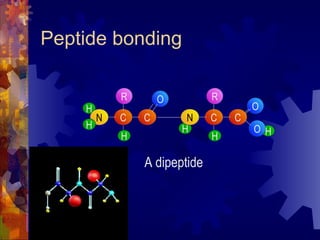
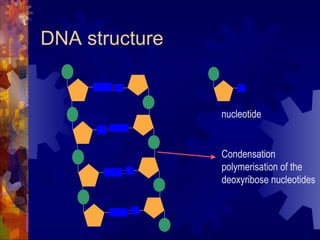
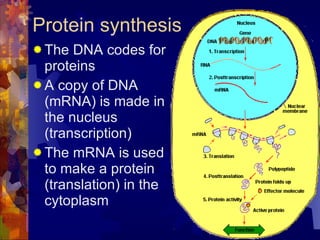
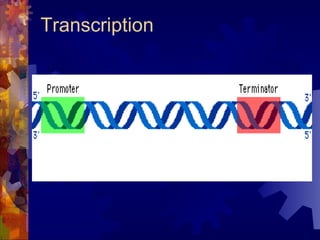
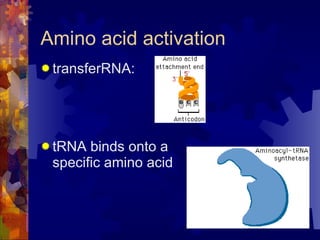
The document provides an overview of key biological molecules including carbohydrates, lipids, proteins, and nucleic acids. It describes the structures and functions of monomers, polymers, and larger molecules formed from these basic units. Key points covered include the structures of monosaccharides, disaccharides, and polysaccharides; fatty acid and triglyceride structures; levels of protein structure from primary to quaternary; and DNA, RNA, and protein synthesis processes.