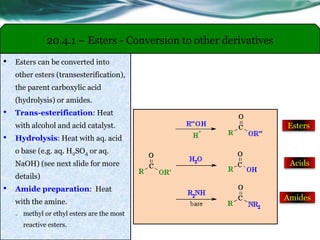
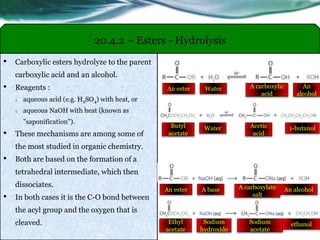
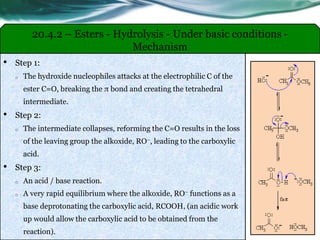
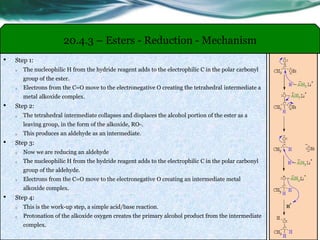
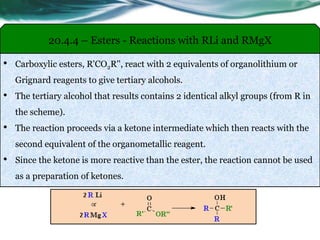
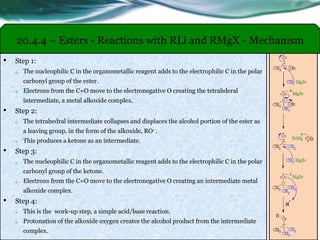
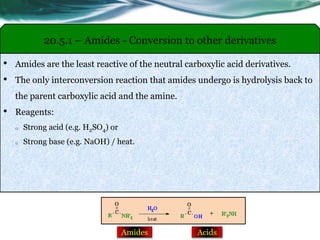
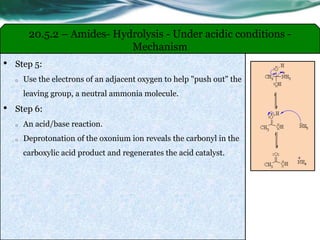
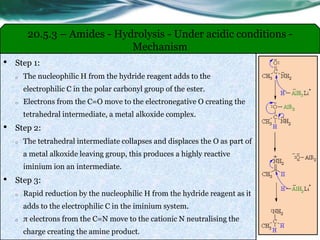
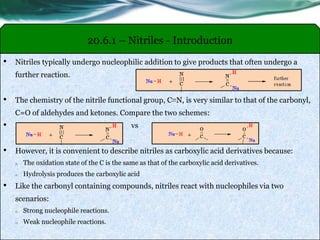
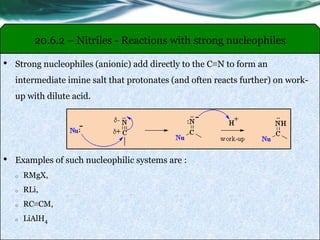
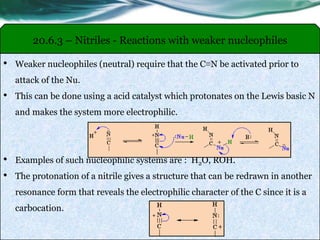
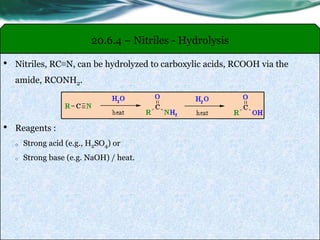
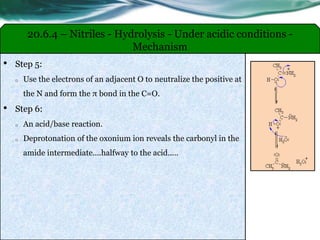
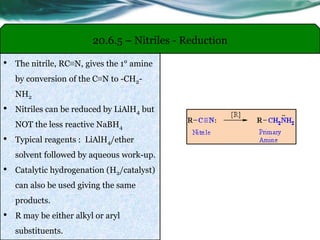
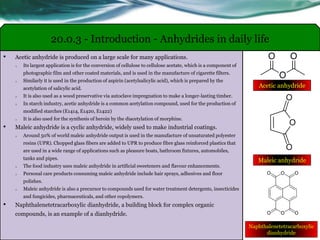
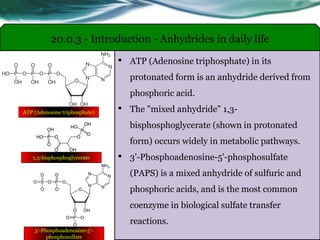
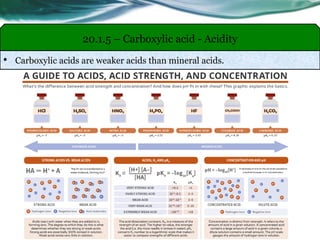
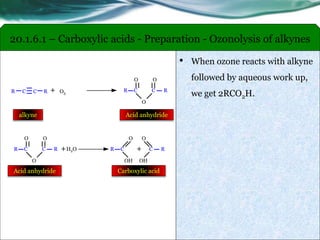
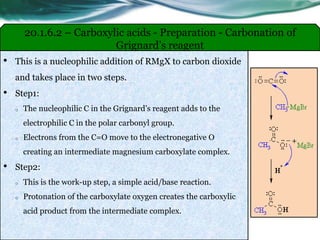
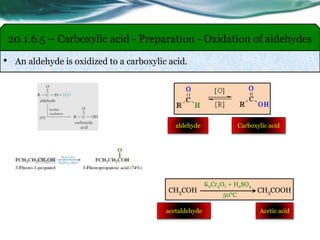
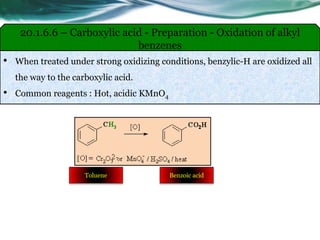
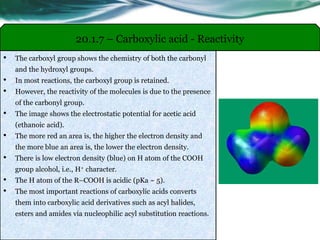
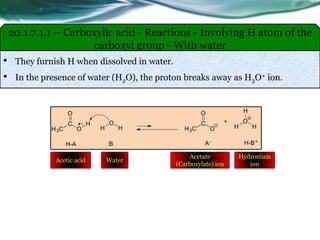
The document provides an overview of carboxylic acids and their derivatives, including their preparation methods, reactivity, isomerism, and applications in various industries such as food, pharmaceuticals, and manufacturing. Specific preparation methods discussed include hydrolysis of nitriles, oxidation of alcohols, and carbonation of Grignard’s reagents, along with the conversion of carboxylic acids into derivatives like esters and amides. The document also highlights the significance of these compounds in health, nutrition, and everyday products.


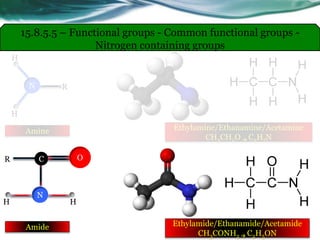
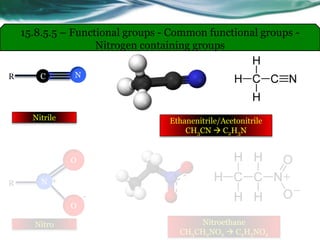


















![20.1.7.3.1 – Carboxylic acid - Reactions - Involving carboxylic
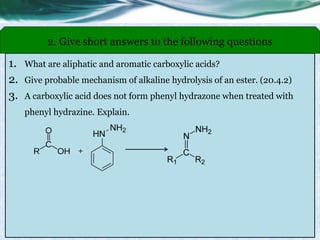
group - Reduction to alcohols
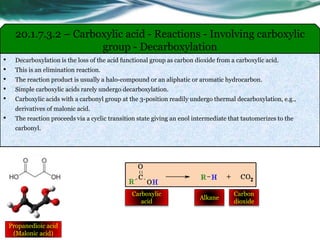
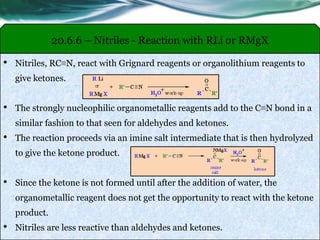
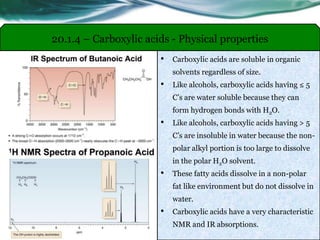
• Carboxylic acids, acid halides, esters, and amides are easily reduced by
strong reducing agents, such as lithium aluminum hydride (LiAlH4).
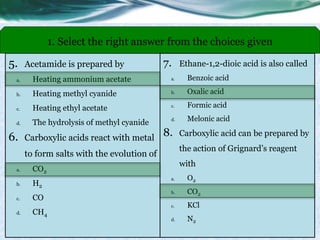
• The carboxylic acids, acid halides, and esters are reduced to alcohols, while
the amide derivative is reduced to an amine.
• Carboxylic acids are less reactive to reduction by hydride than aldehydes,
ketones and esters.
• Carboxylic acids are reduced to primary alcohols.
• As a result of their low reactivity, carboxylic acids can only be reduced by
LiAlH4 to form primary alcohol.
CH3COOH + 4[H] CH3CH2OH + H2O
Acetic acid ethanol
LiAlH4](https://image.slidesharecdn.com/chapter20-carboxylicacidsandfunctionalderivatives-200329001217/85/Chapter-20-carboxylic-acids-and-functional-derivatives-53-320.jpg)