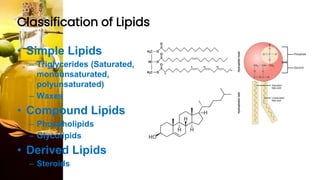
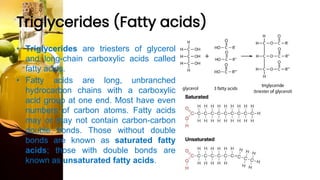
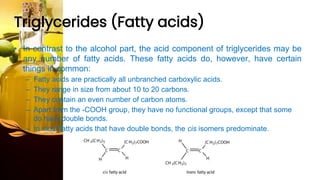
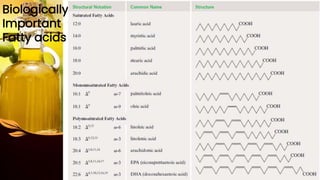
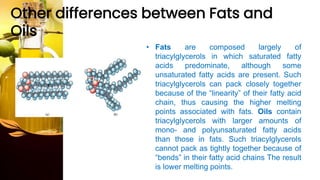
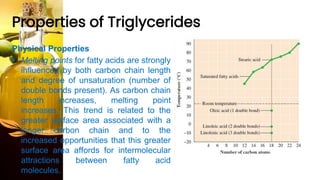
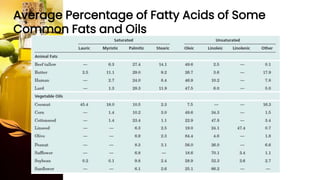
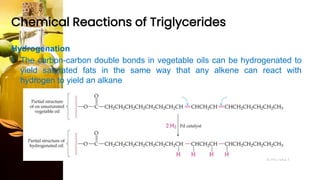
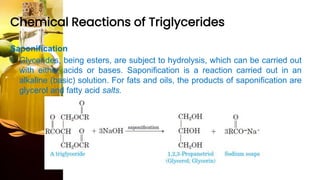
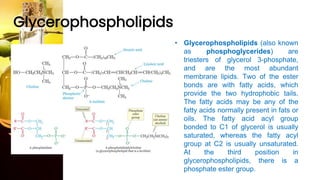
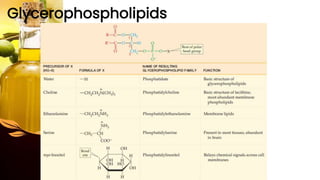
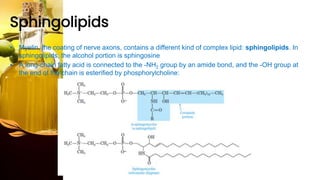
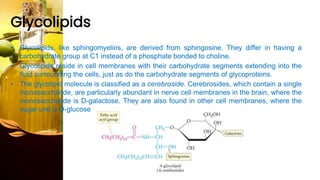
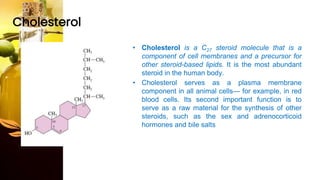
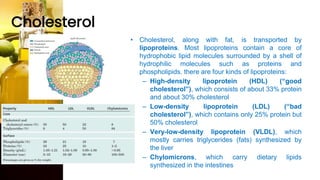
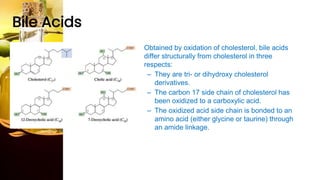
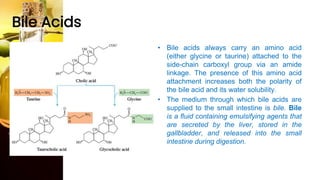
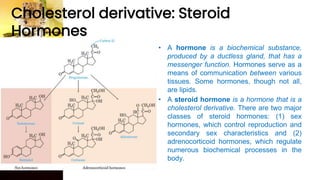
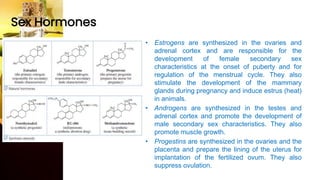
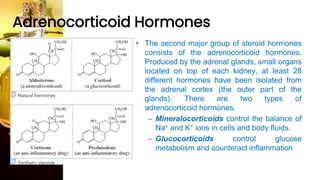
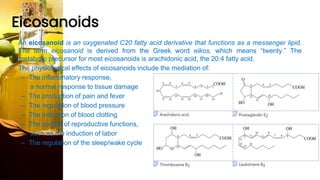
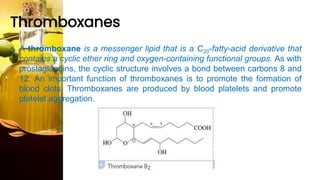
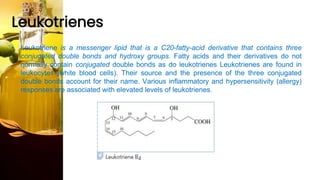
This document provides information on lipids, including their classification, functions, and examples. It discusses simple lipids like triglycerides and fatty acids, as well as complex lipids including phospholipids, glycolipids, sterols, and steroids. Specific lipid types and their structures are explained, such as cholesterol, bile acids, eicosanoids like prostaglandins, and steroid hormones. Lipids serve important roles in the body including energy storage, membrane components, and cell signaling.