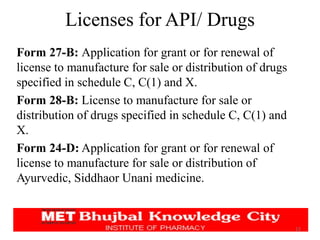
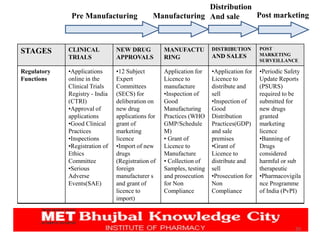
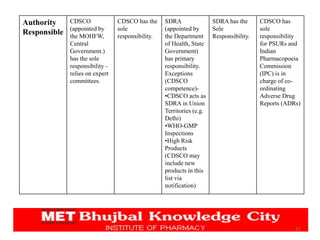
The document discusses the pharmaceutical industry development process in India. It outlines the legal requirements and licenses needed to manufacture or import APIs and drugs. Companies must seek approval from the DCGI and adhere to CDSCO guidelines. The application process requires submitting chemical, pharmaceutical, pre-clinical and clinical data. Various forms are used for obtaining manufacturing, import, and sales licenses from the CDSCO. The CDSCO-SUGAM project aims to streamline approval processes.