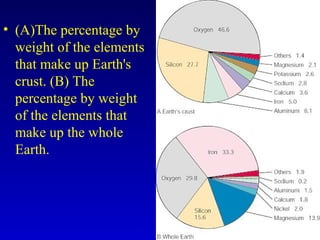
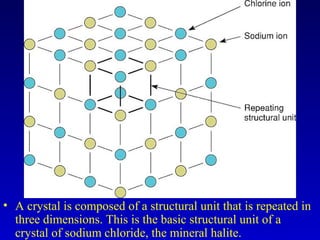
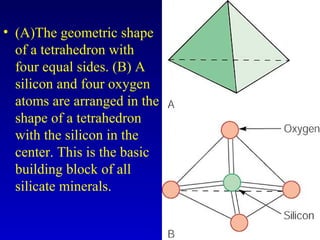
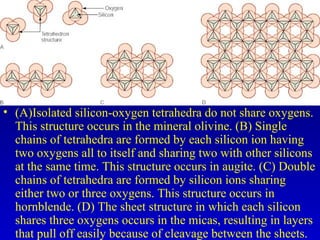
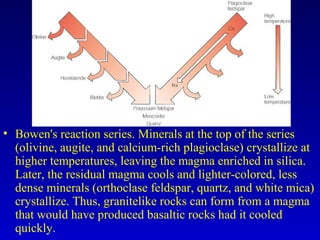
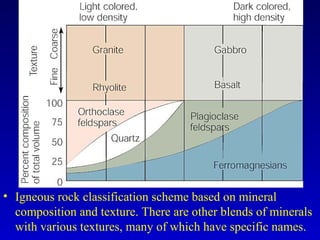
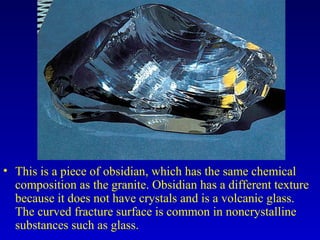
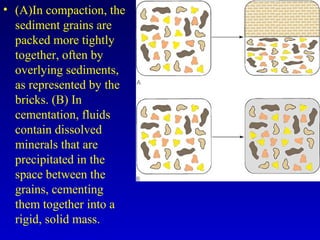
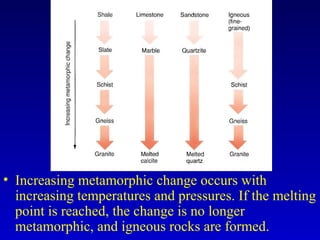
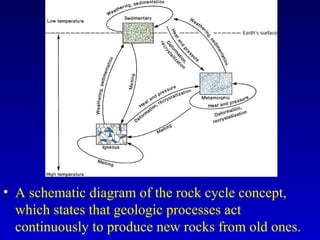
This document provides information about rocks and minerals. It discusses how Earth's early molten stage led to differentiation of the crust. It also explains that minerals have unique crystalline structures while rocks are aggregates of minerals. The main rock types - igneous, sedimentary and metamorphic - are formed by different geological processes. Igneous rocks form from cooling magma, sedimentary rocks form through compaction and cementation, and metamorphic rocks form through heat and pressure altering existing rocks.