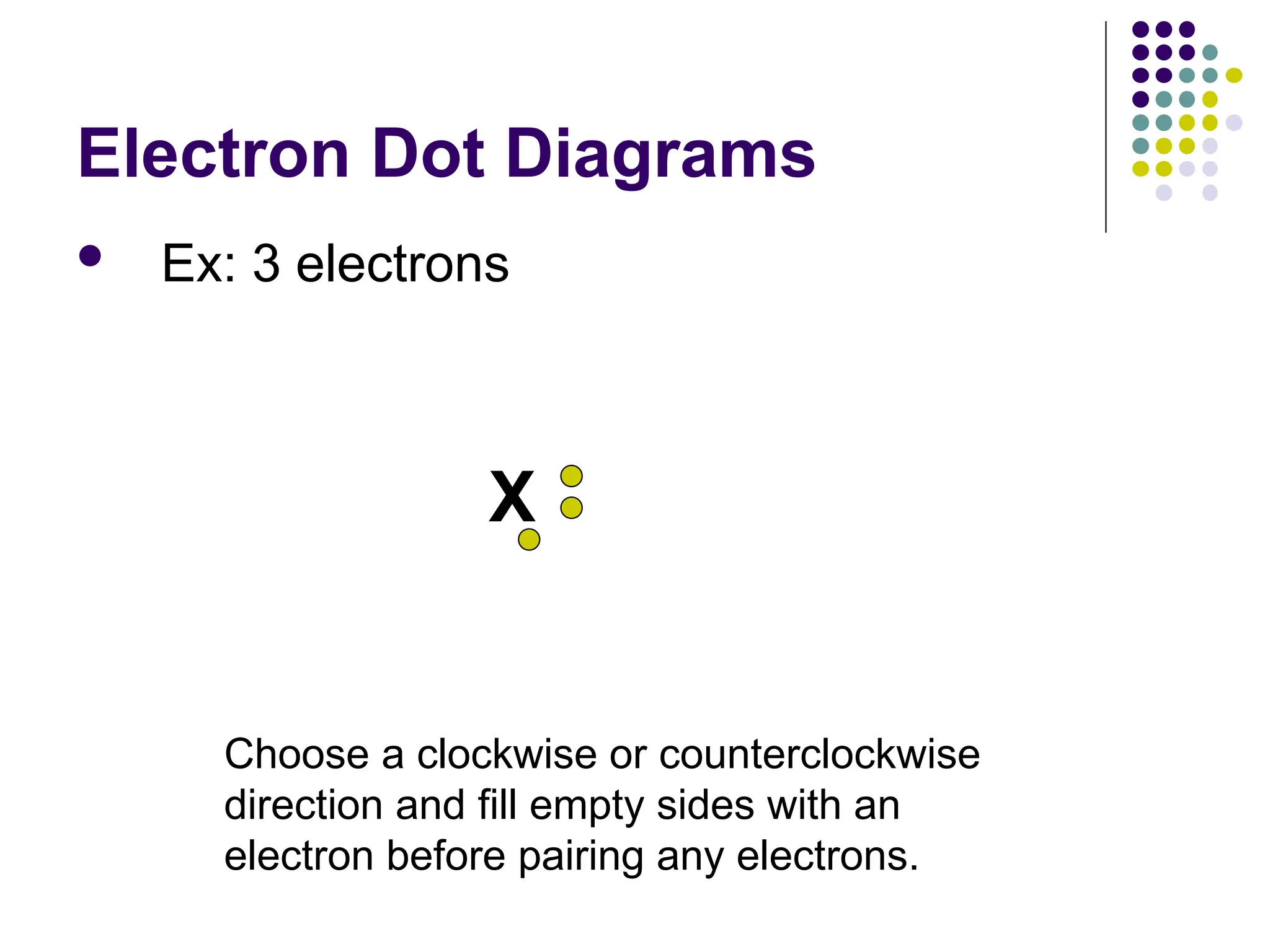
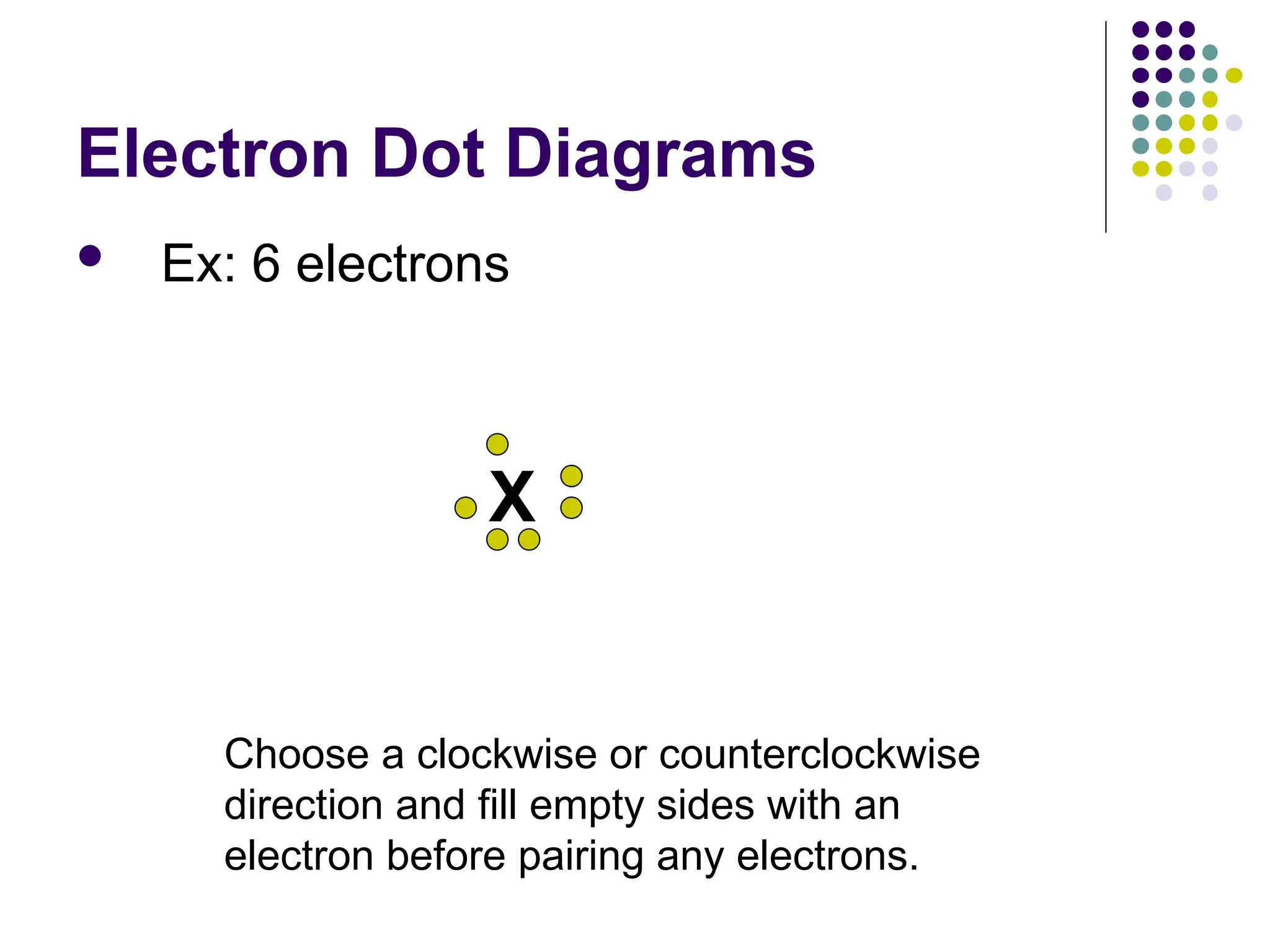
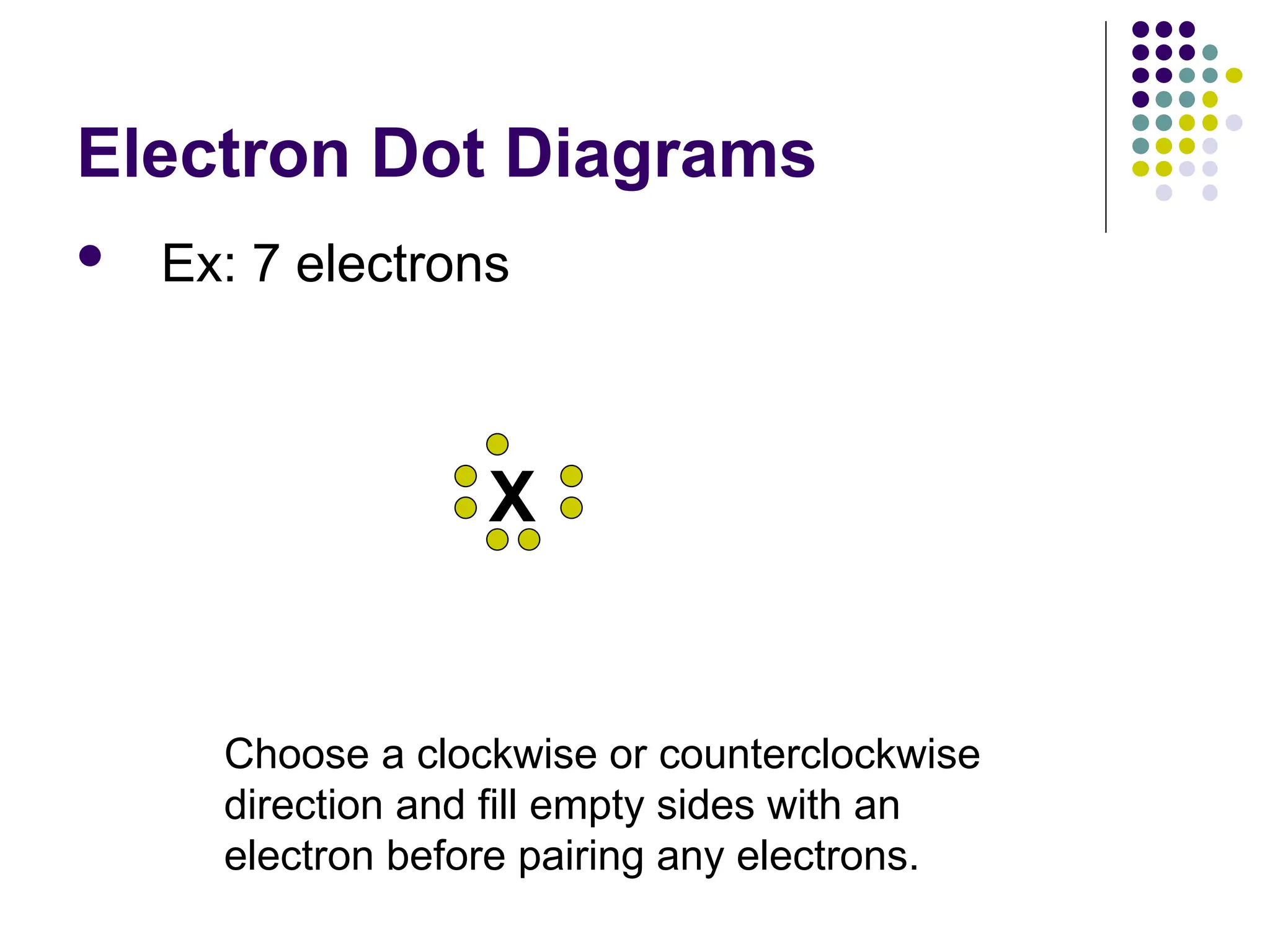
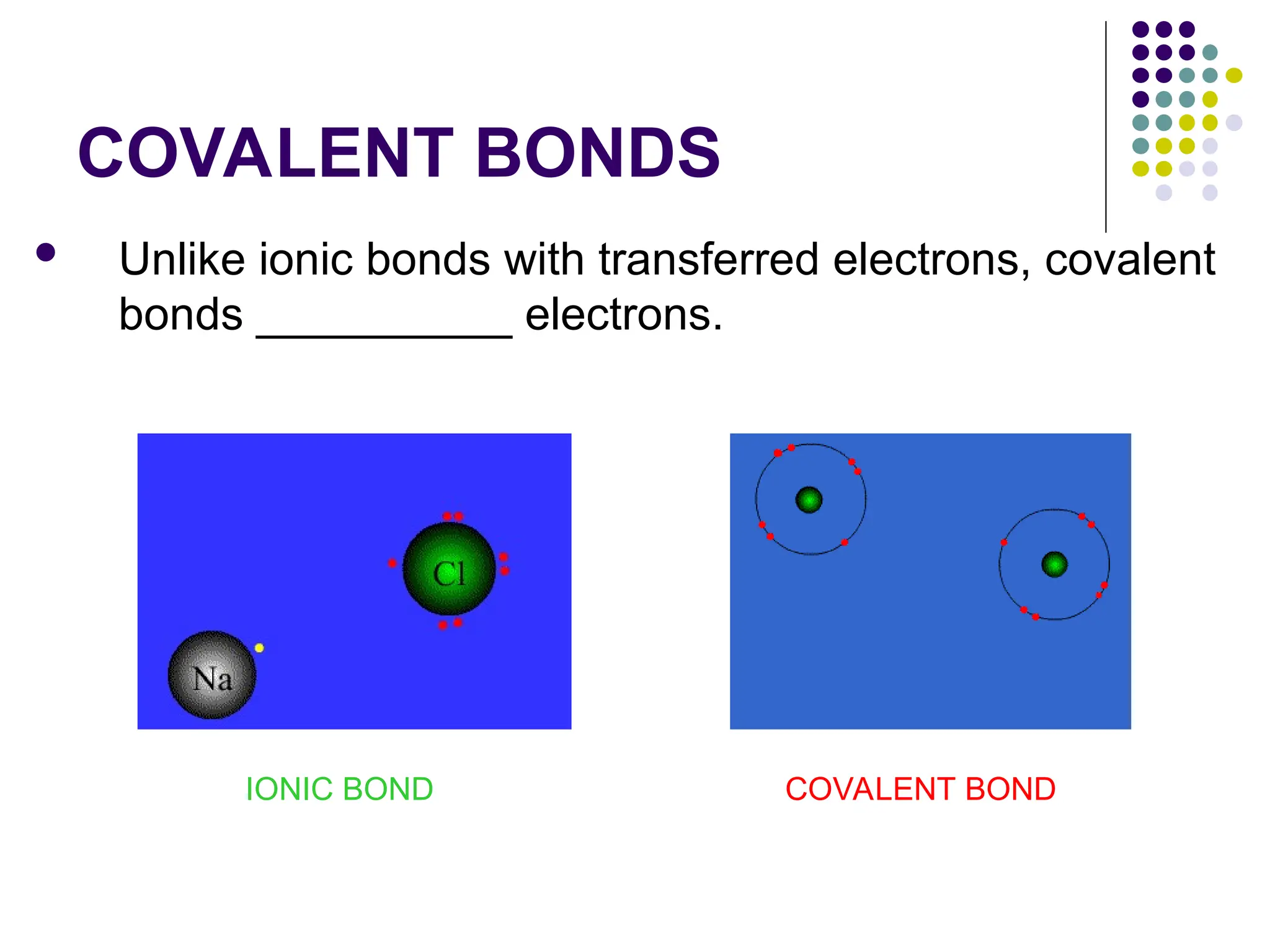
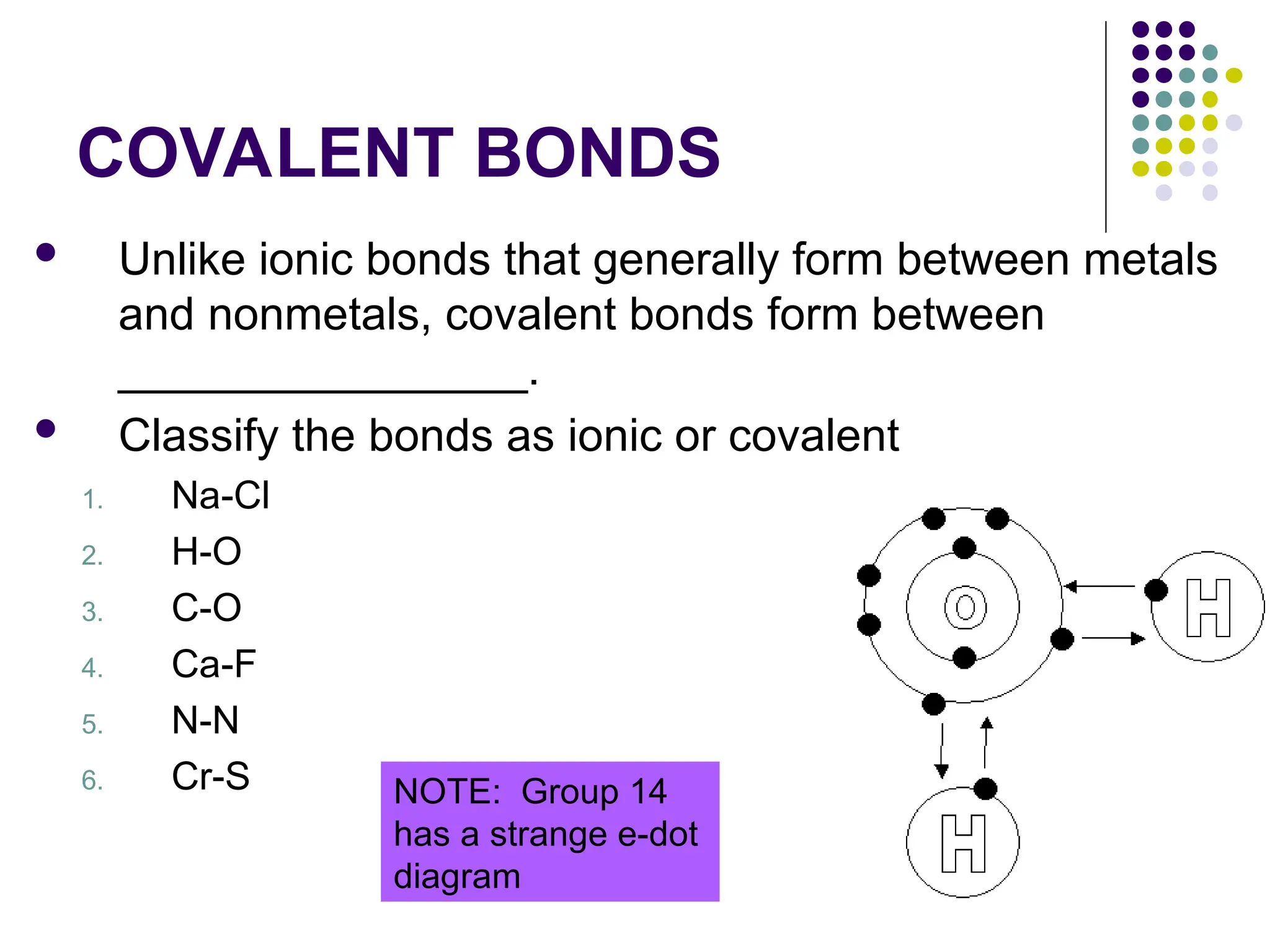
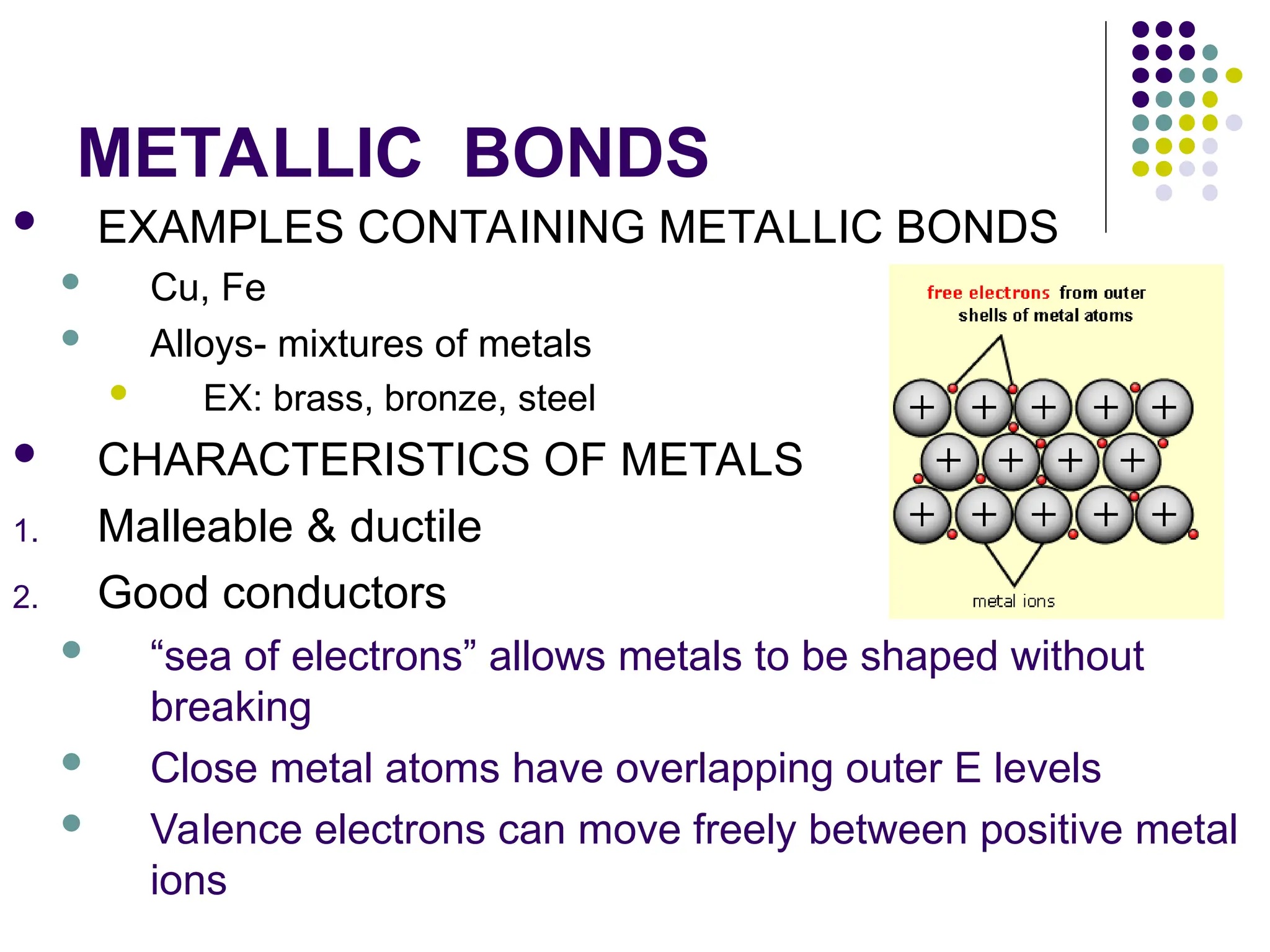
The document discusses chemical bonding and interactions of matter, focusing on the identification and arrangement of electrons in various atoms using electron dot diagrams. It explains different types of bonds, including ionic, covalent, and metallic bonds, along with their characteristics and how to name associated compounds. Additionally, it highlights properties of ionic and covalent compounds, such as conductivity, melting points, and mixtures of elements.