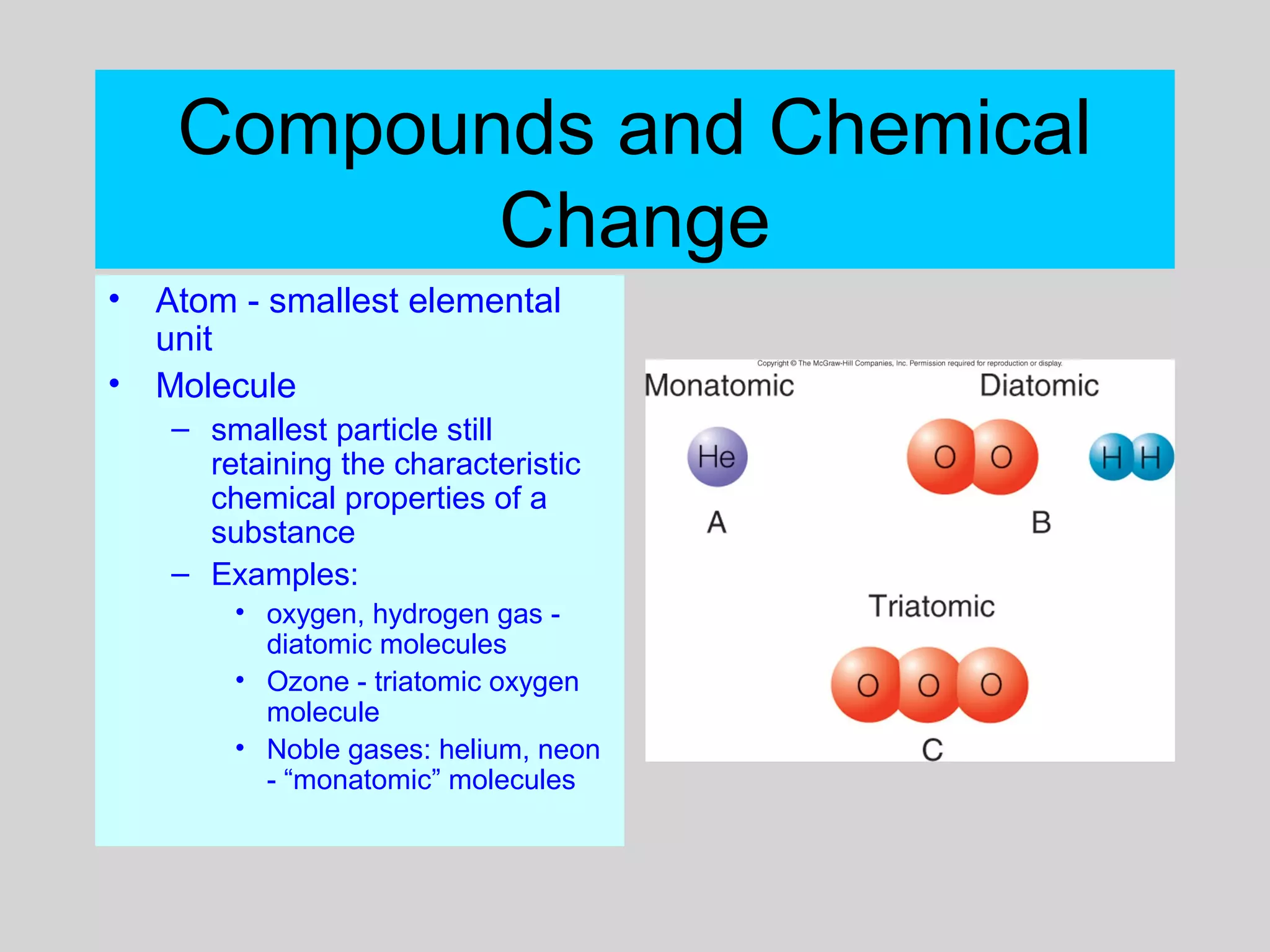
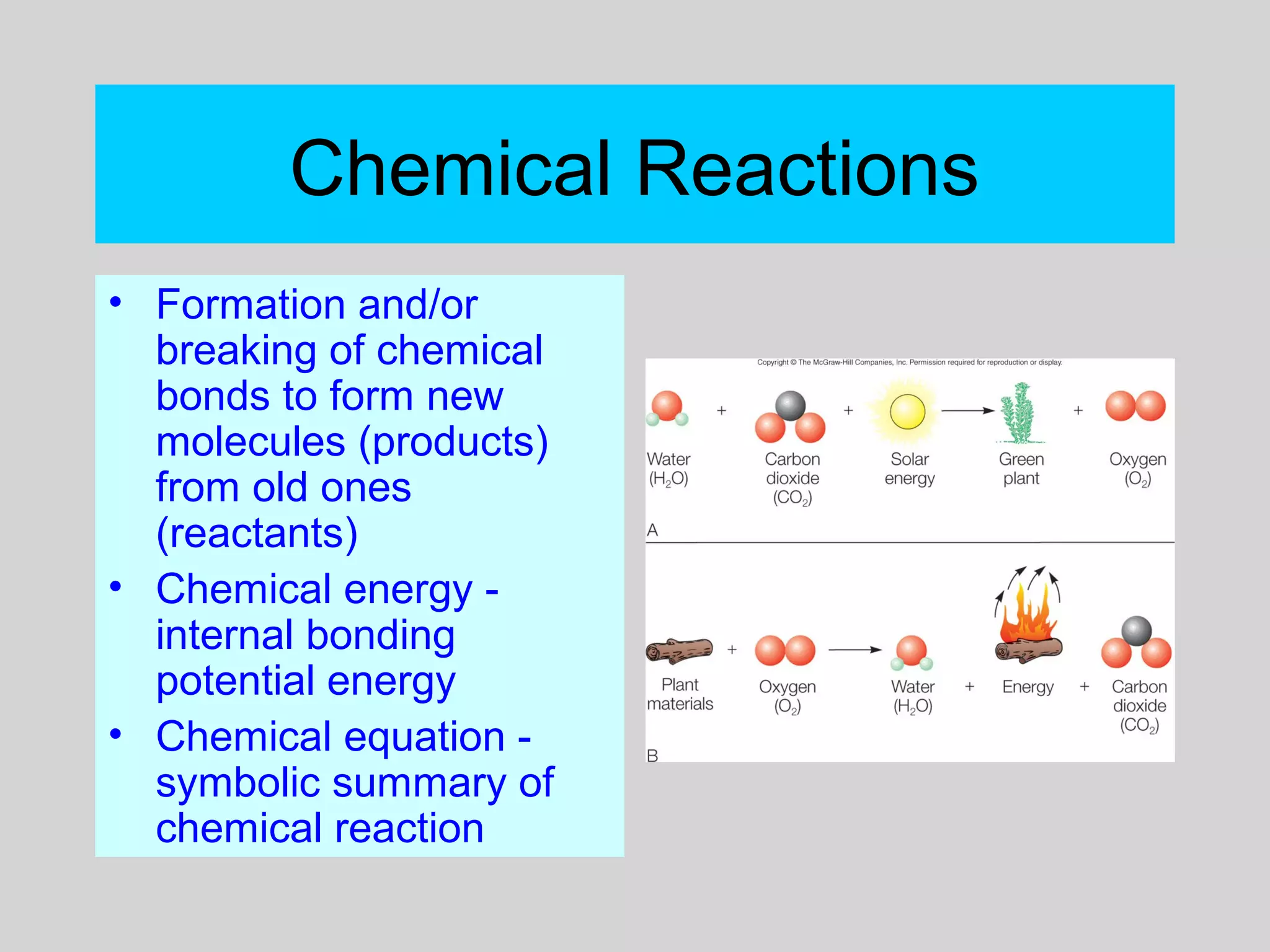
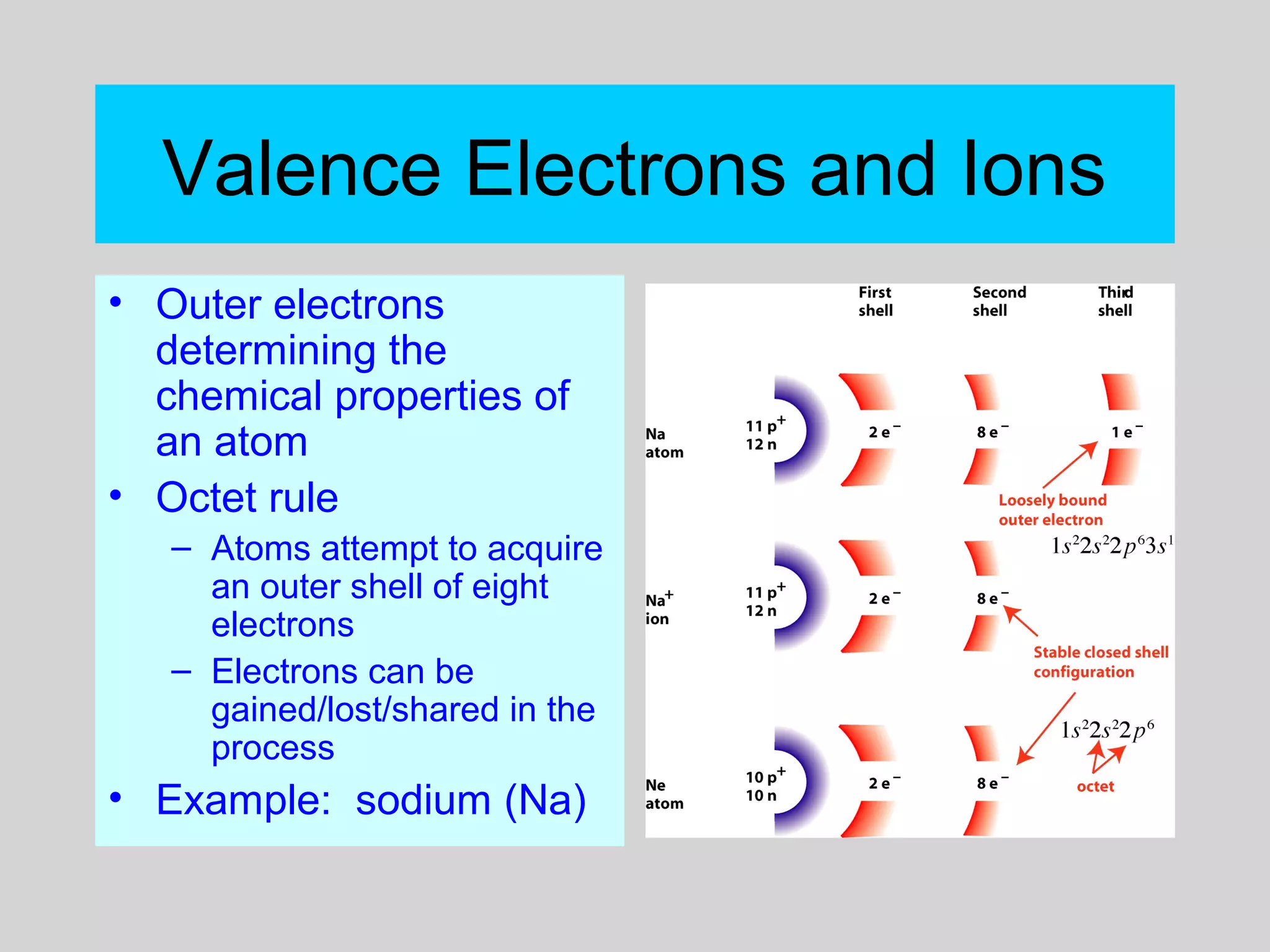
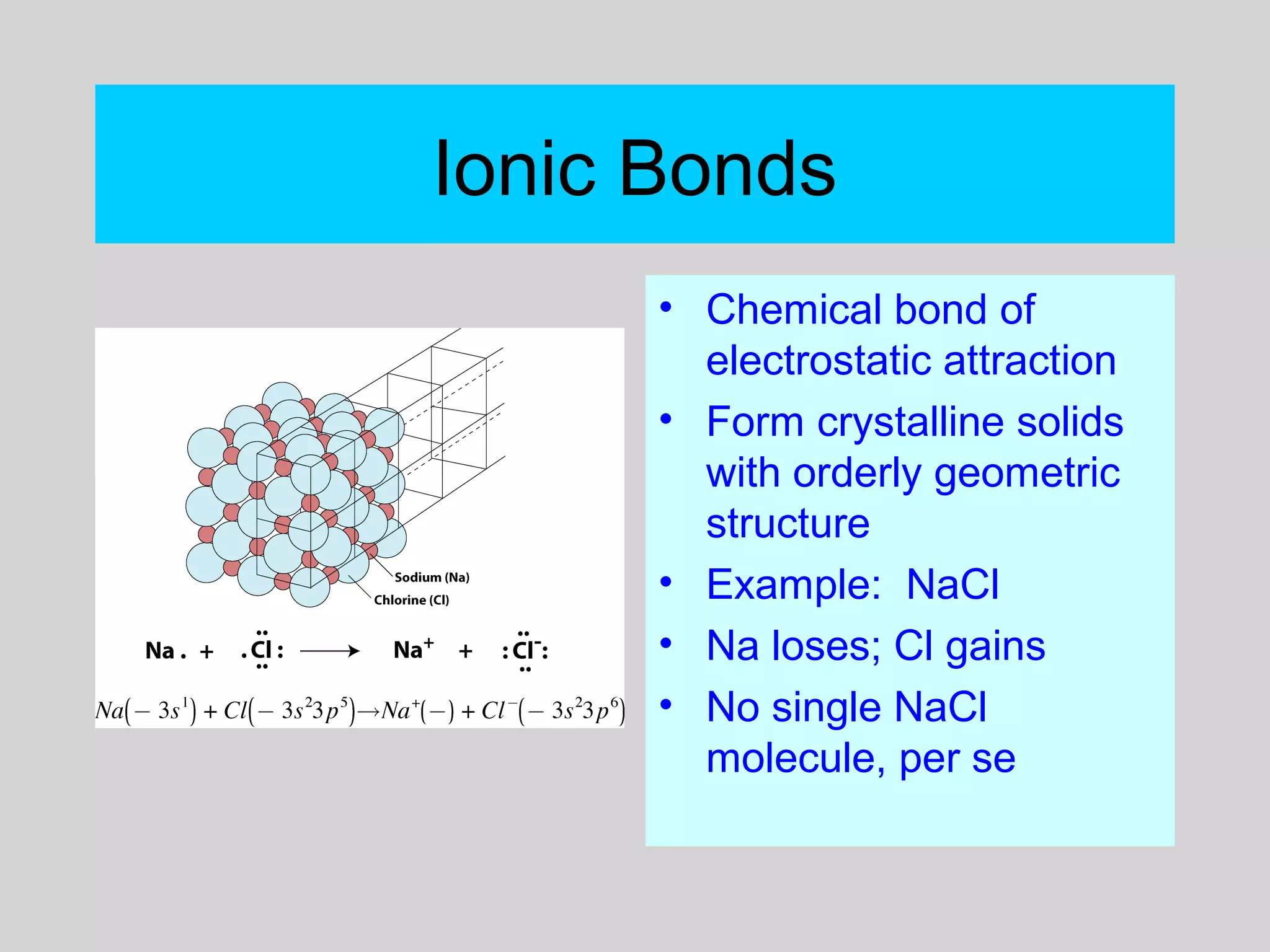
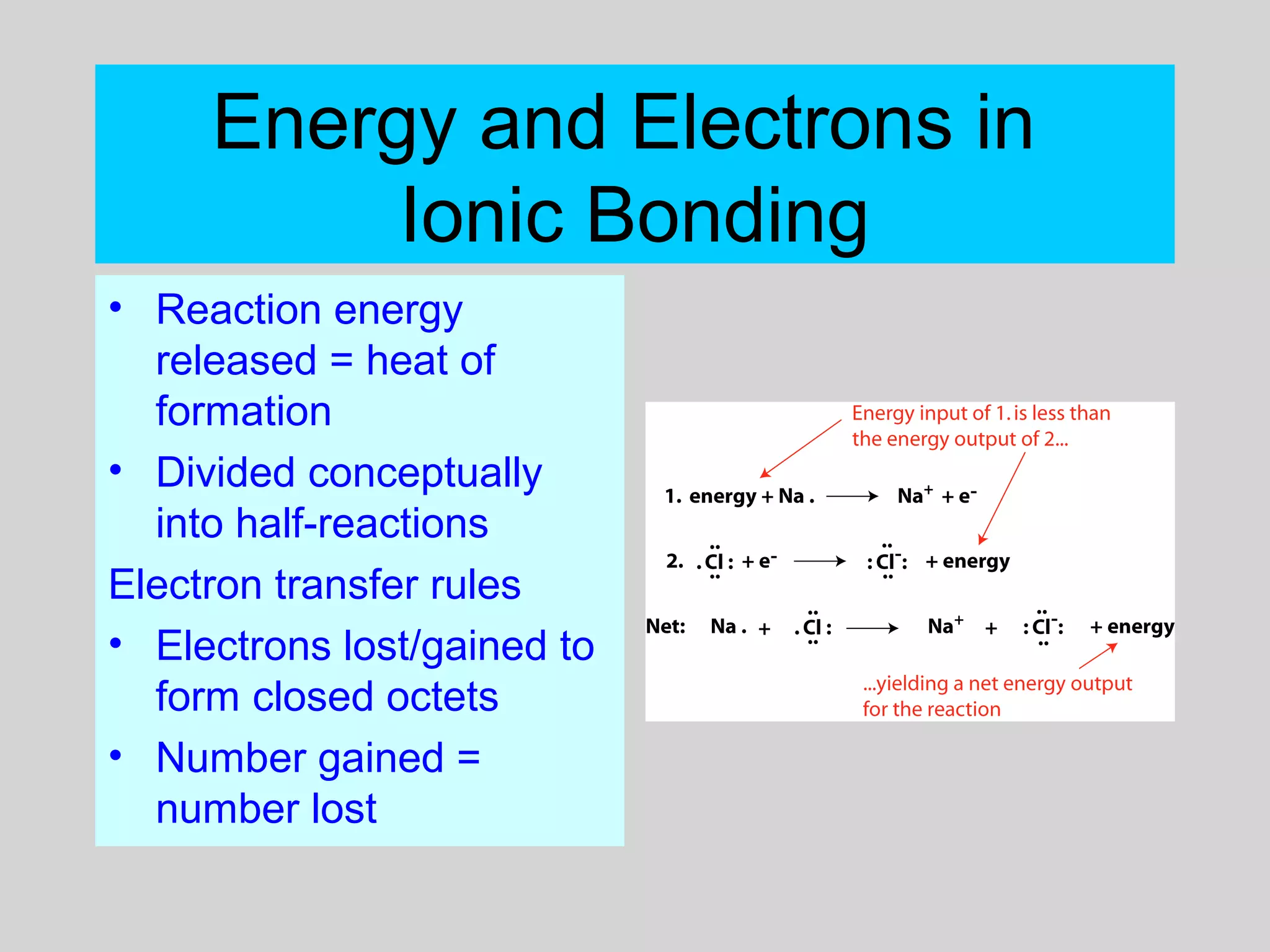
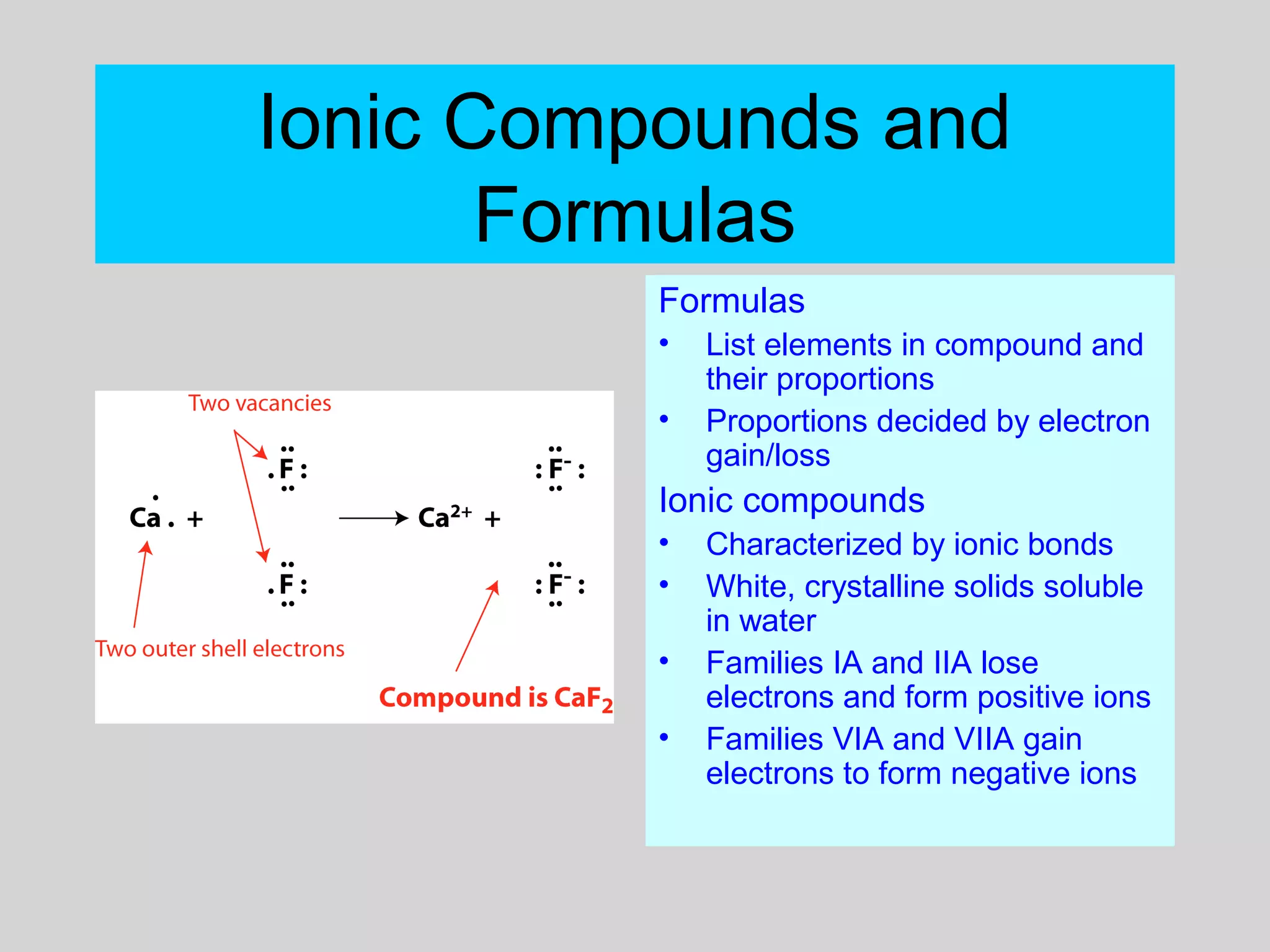
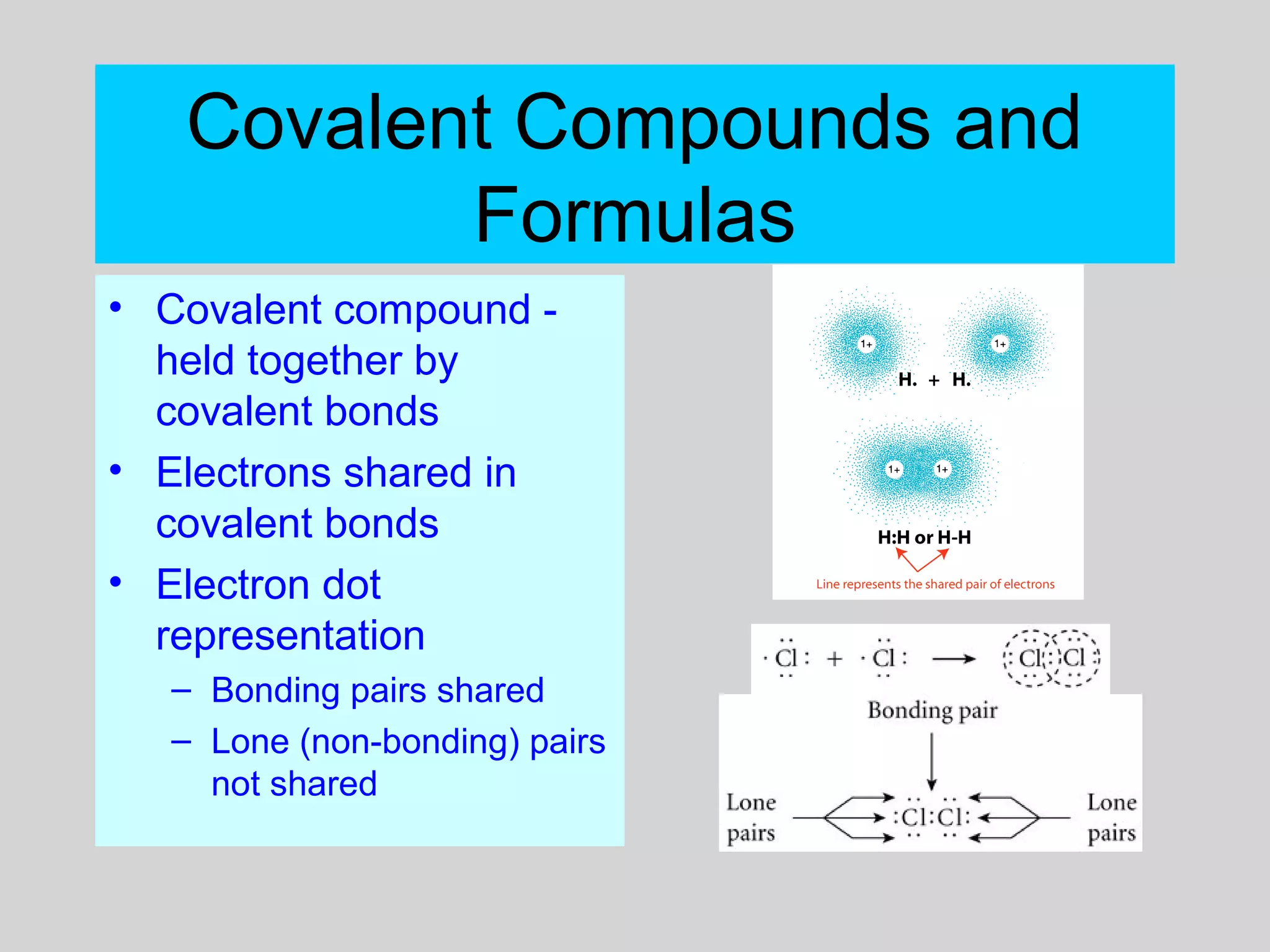
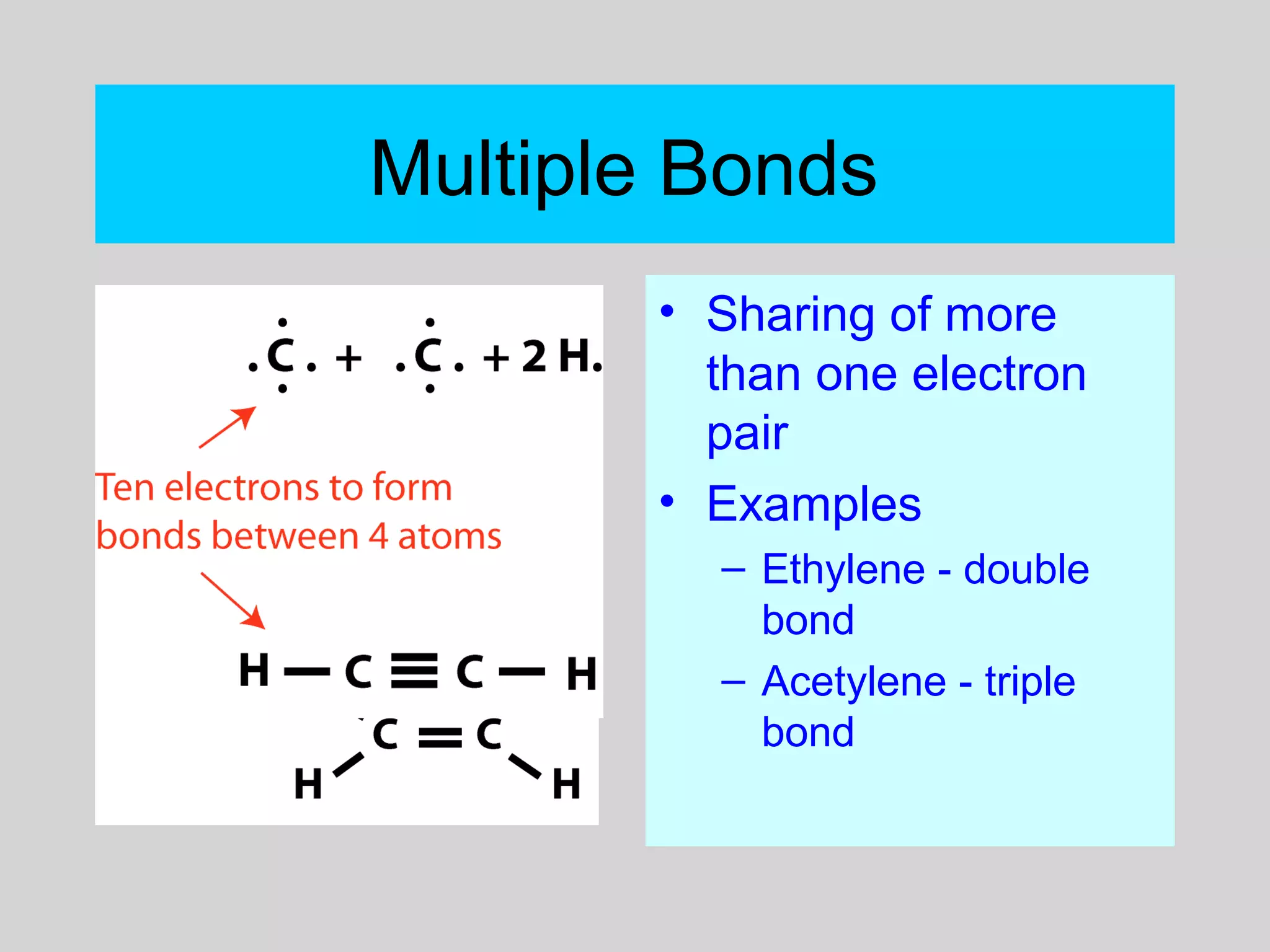
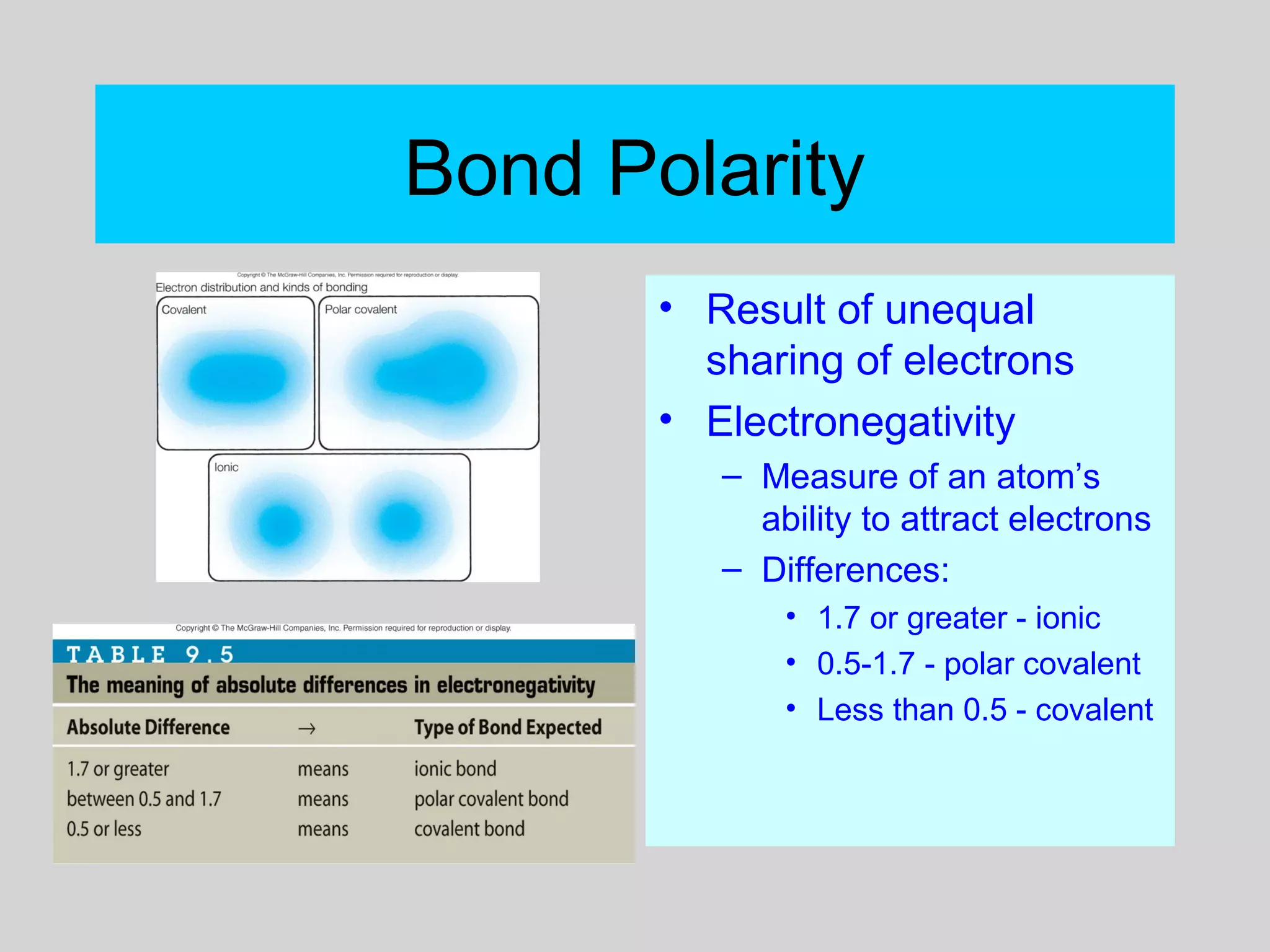
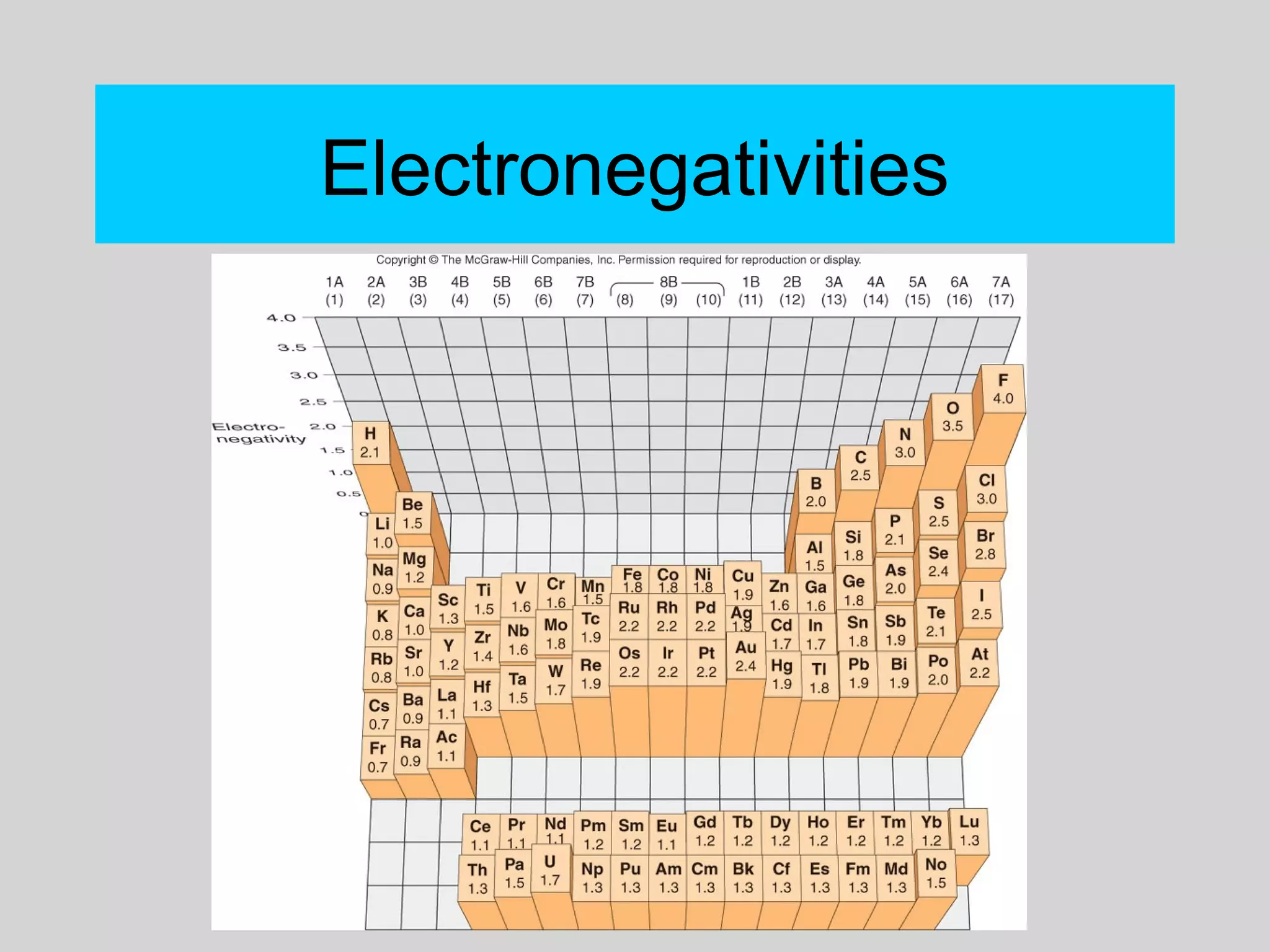
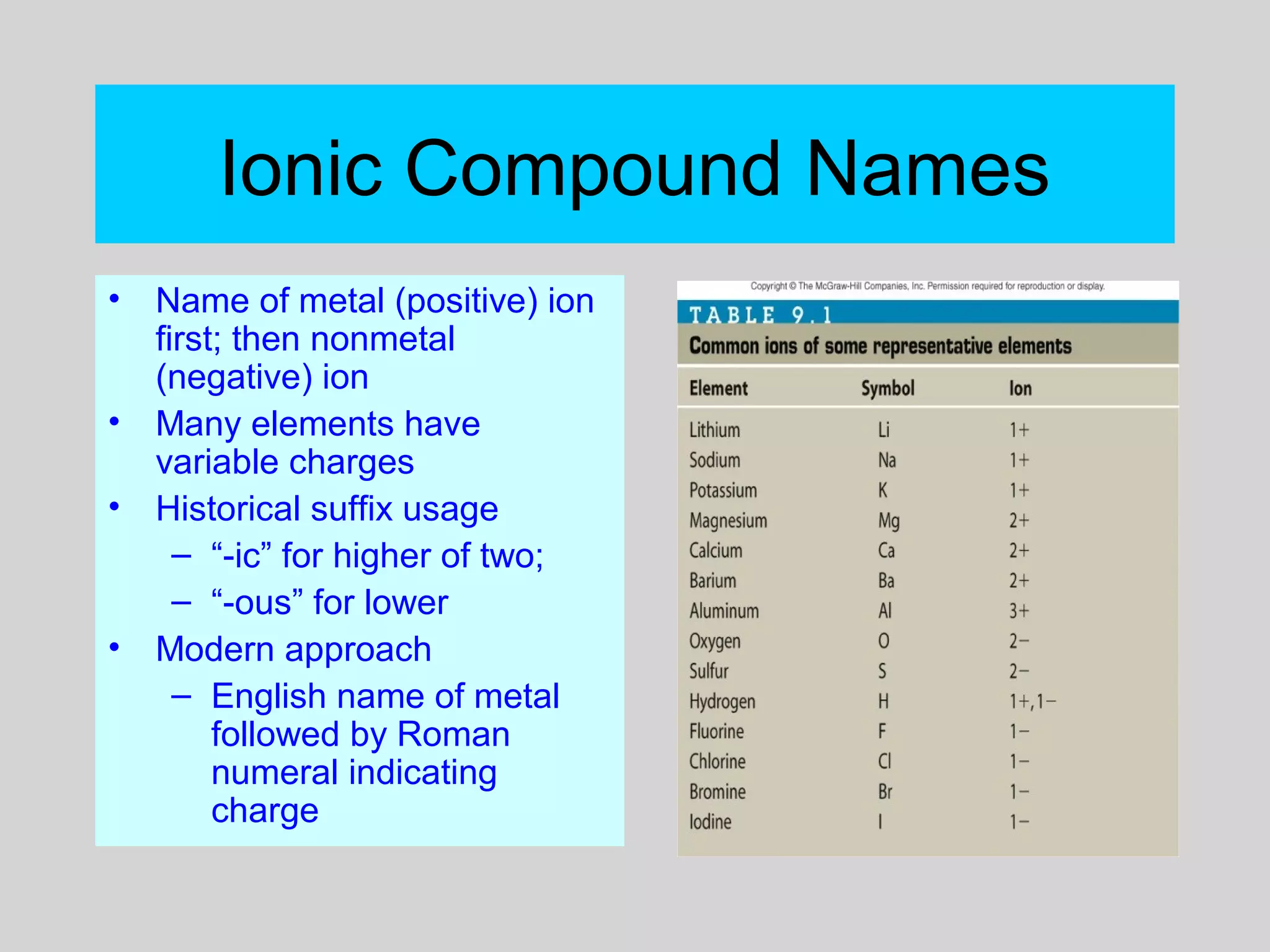
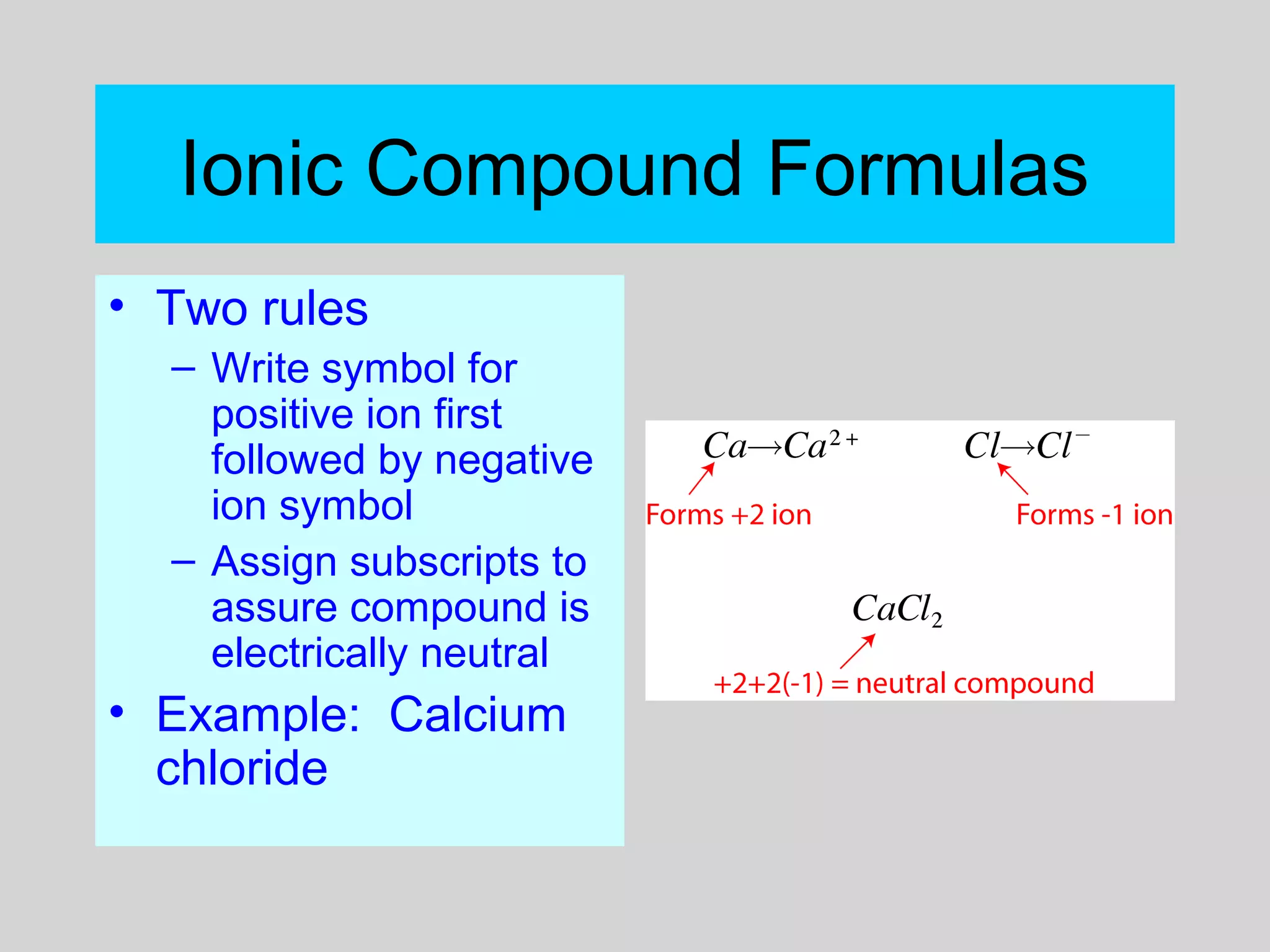
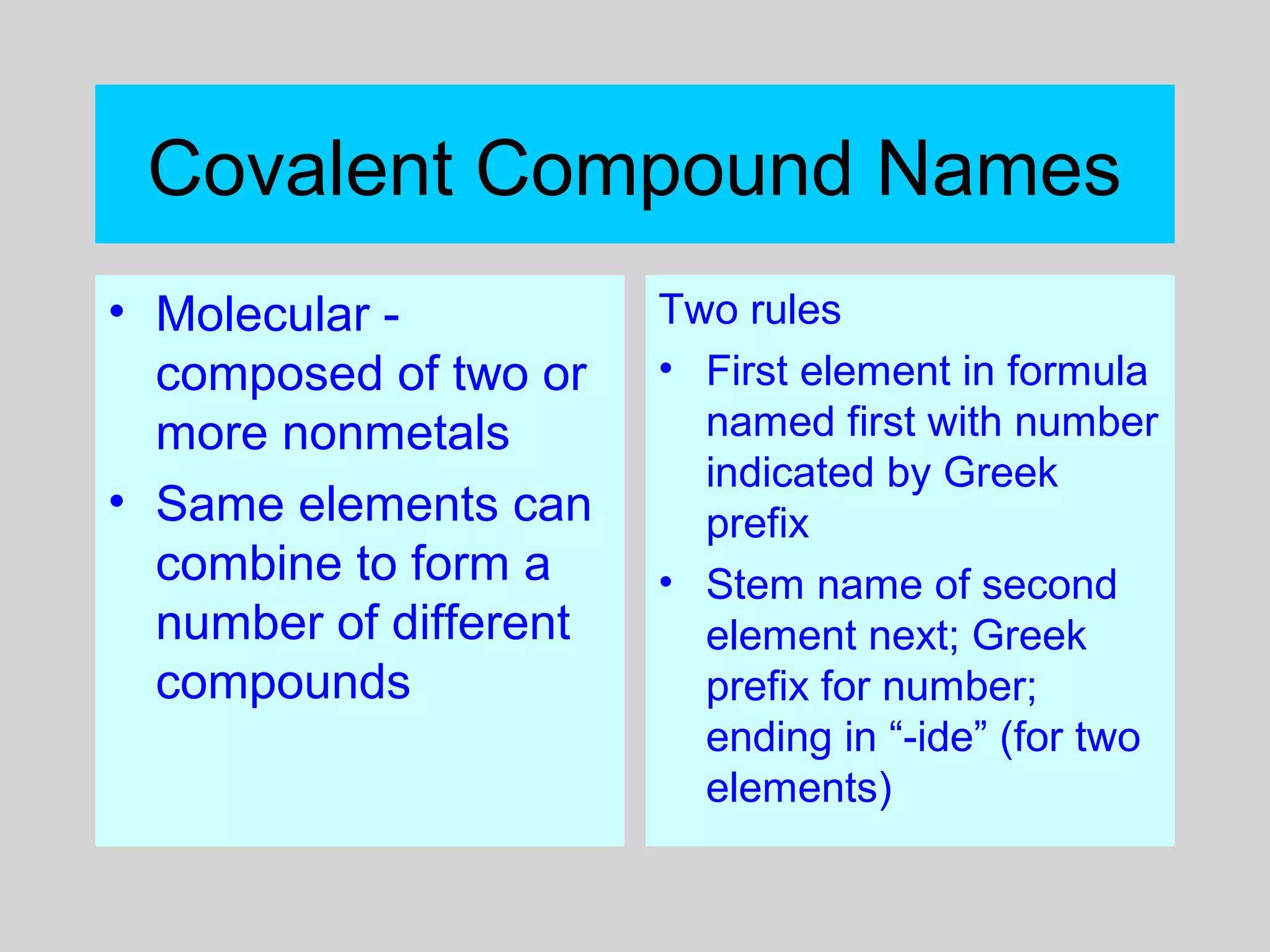
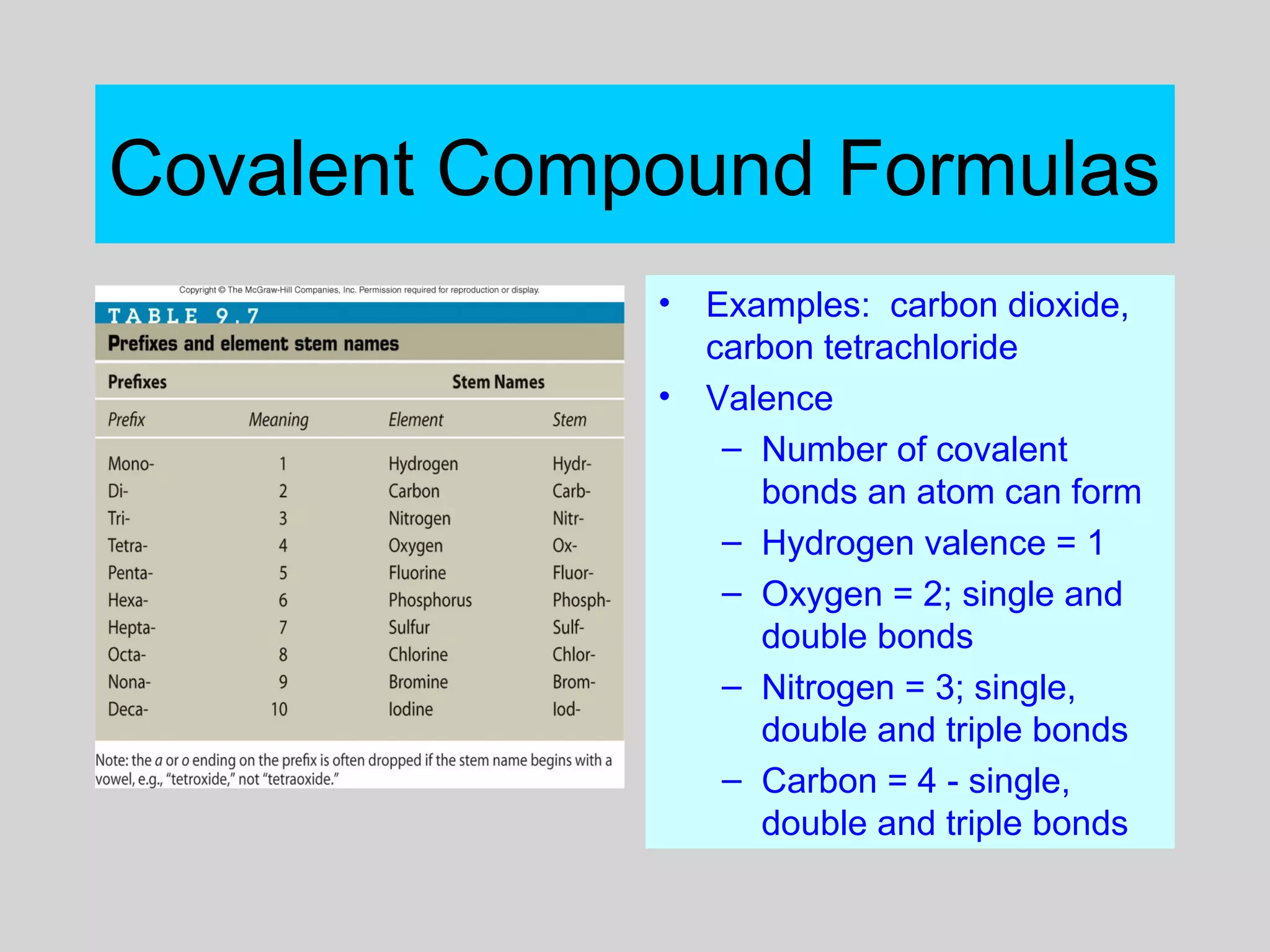
This document discusses chemical bonds and how they form compounds. It explains that there are three main types of bonds: ionic, covalent, and metallic. Ionic bonds form when electrons are transferred between atoms. Covalent bonds form when atoms share pairs of electrons to achieve stable electron configurations. Metallic bonds involve delocalized electrons that are shared throughout the metal. The document also discusses naming and writing formulas for ionic and covalent compounds according to systematic rules based on the elements present and their bonding structure.