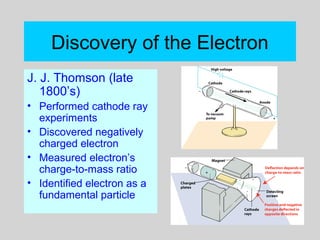
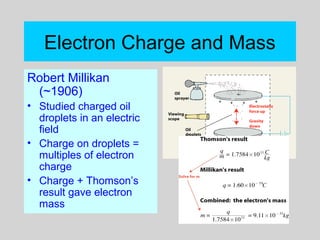
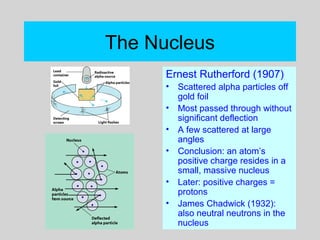
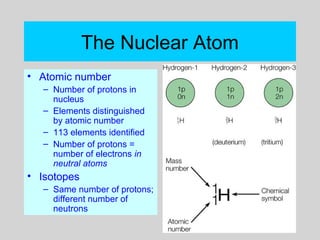
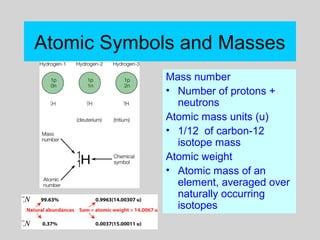
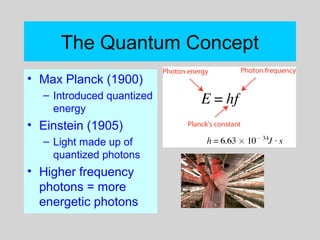
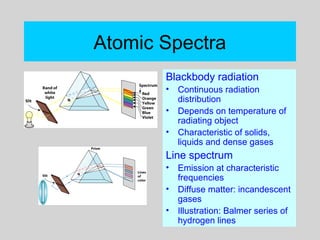
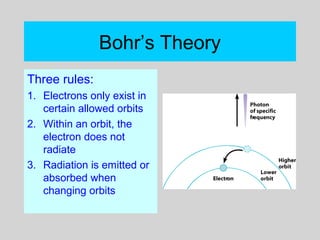
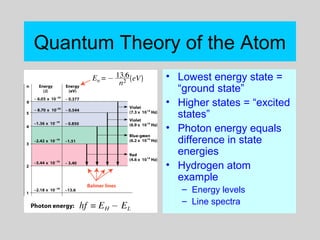
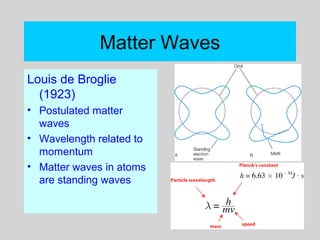
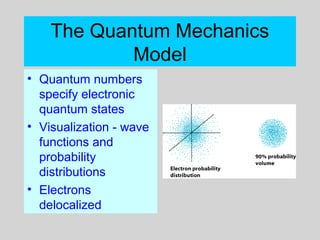
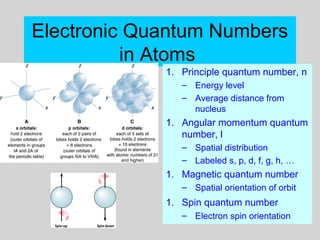
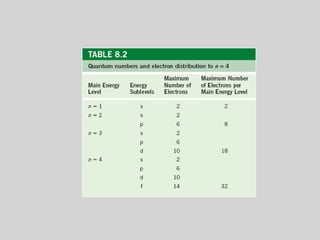
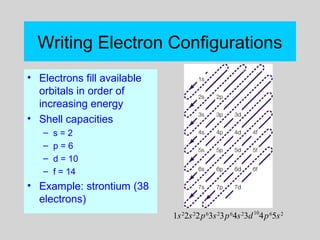
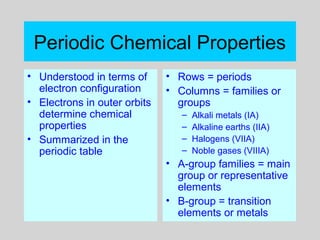
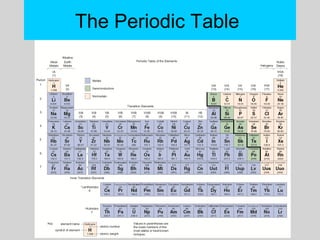
This document discusses the development of atomic theory from ancient Greek philosophers to the modern quantum mechanical model. Key contributors and discoveries include Dalton formulating the first atomic theory, Thomson discovering the electron, Rutherford deducing the nuclear atom from alpha particle scattering experiments, and Bohr, de Broglie, Schrodinger, and others developing quantum mechanics to explain atomic structure and spectra. The modern atomic model consists of electrons in quantized energy levels around an atomic nucleus composed of protons and neutrons, which is summarized by the periodic table and determines chemical properties.