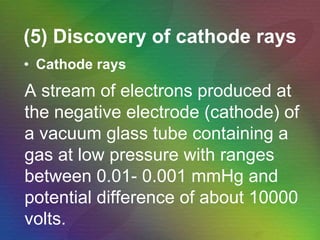
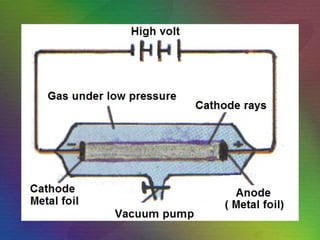
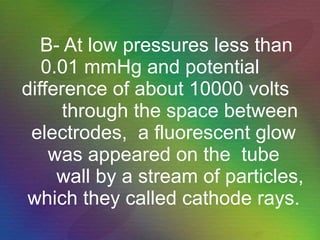
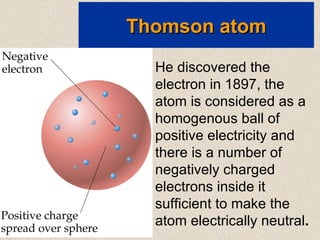
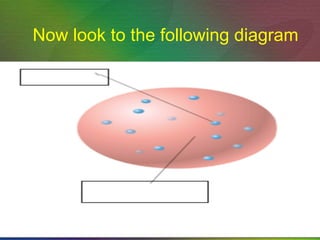
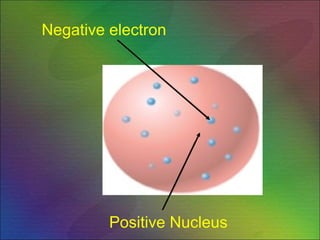
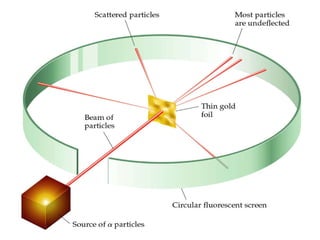
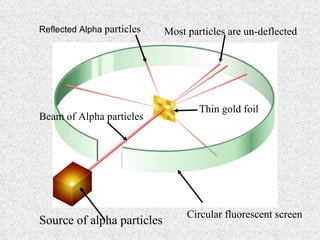
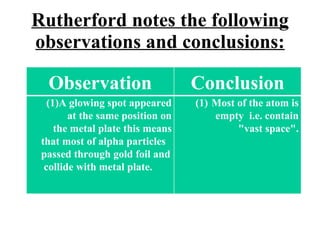
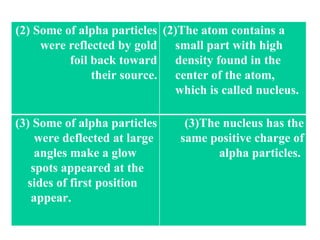
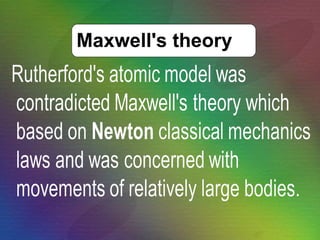
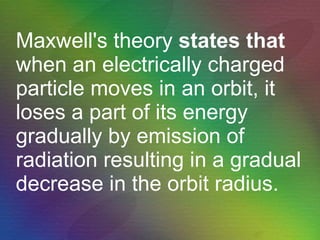
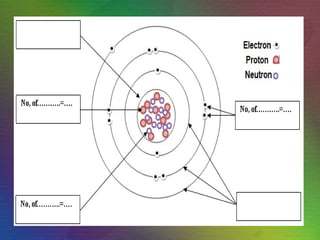
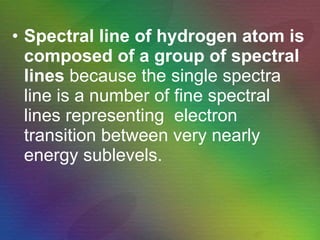
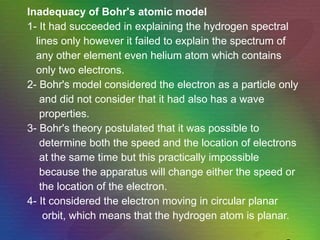
The document outlines the historical evolution of atomic theory, starting from ancient Greek philosophers' ideas about matter to modern understandings involving atomic structure. Key figures like Aristotle, Boyle, Dalton, Thomson, Rutherford, and Bohr made significant contributions, including the discovery of cathode rays, the structure of the atom, and quantum mechanics. It highlights the development of atomic models, their successes, and the limitations of Bohr's model in explaining the spectra of elements beyond hydrogen.