Embed presentation
Download as PDF, PPTX



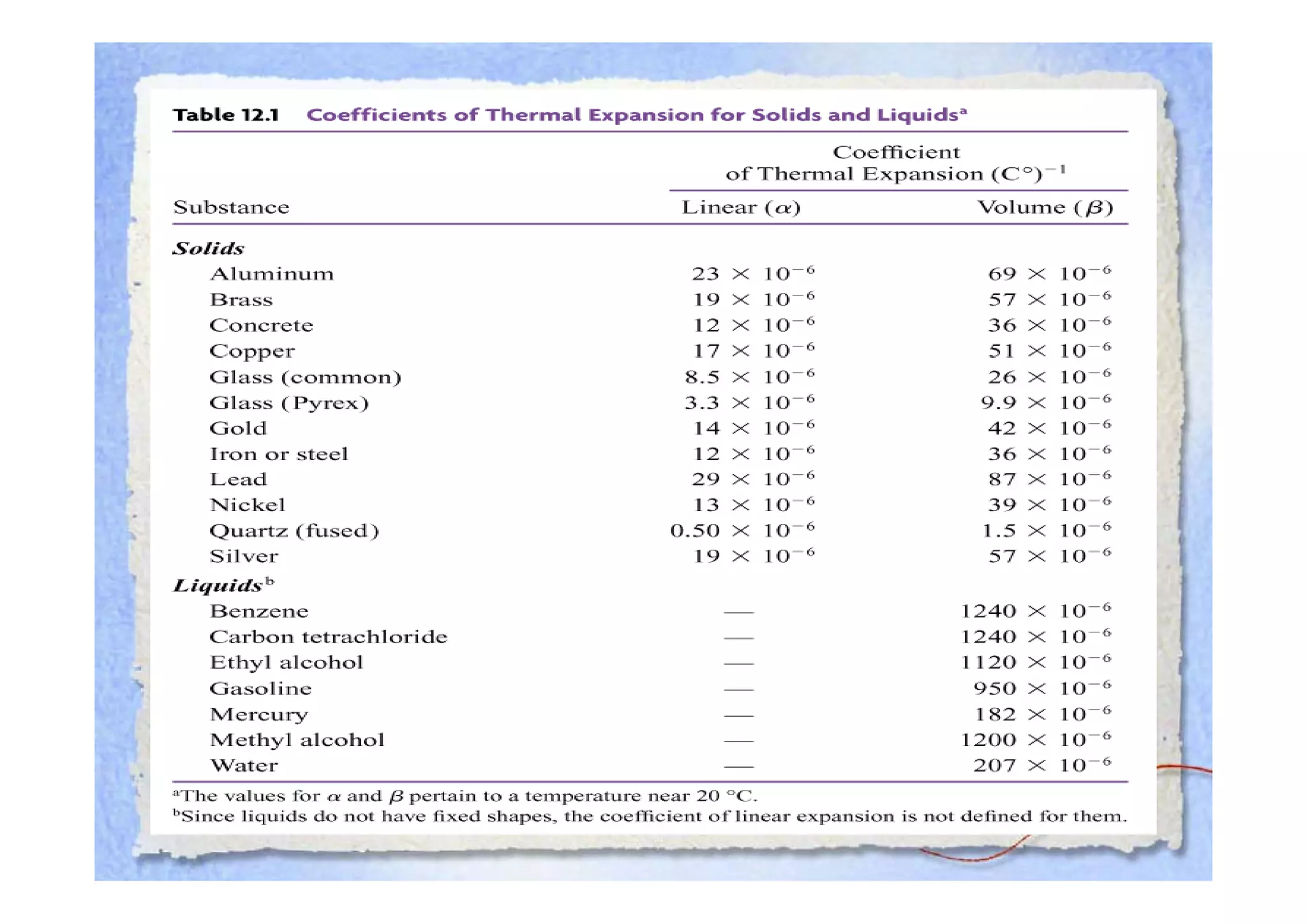

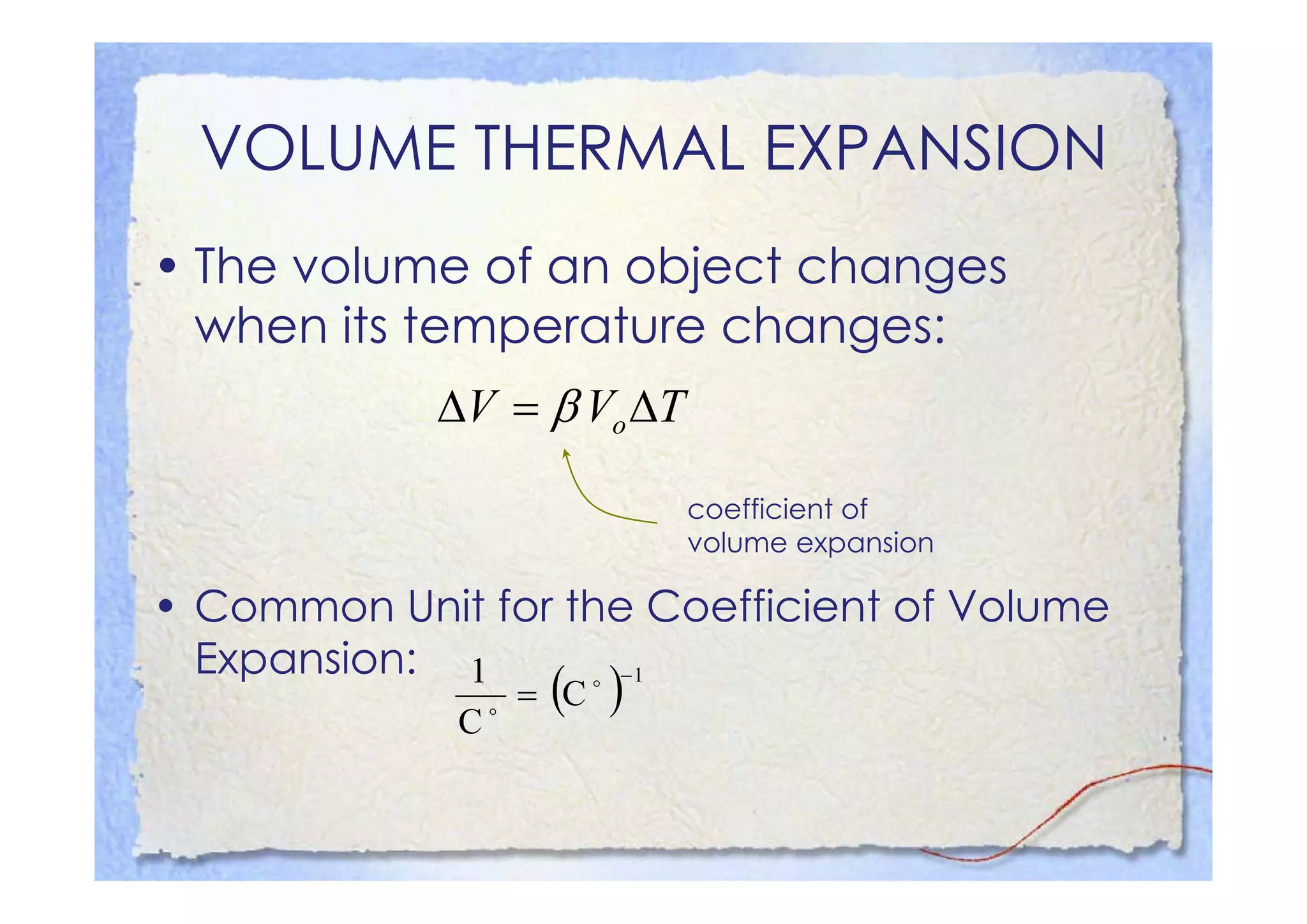



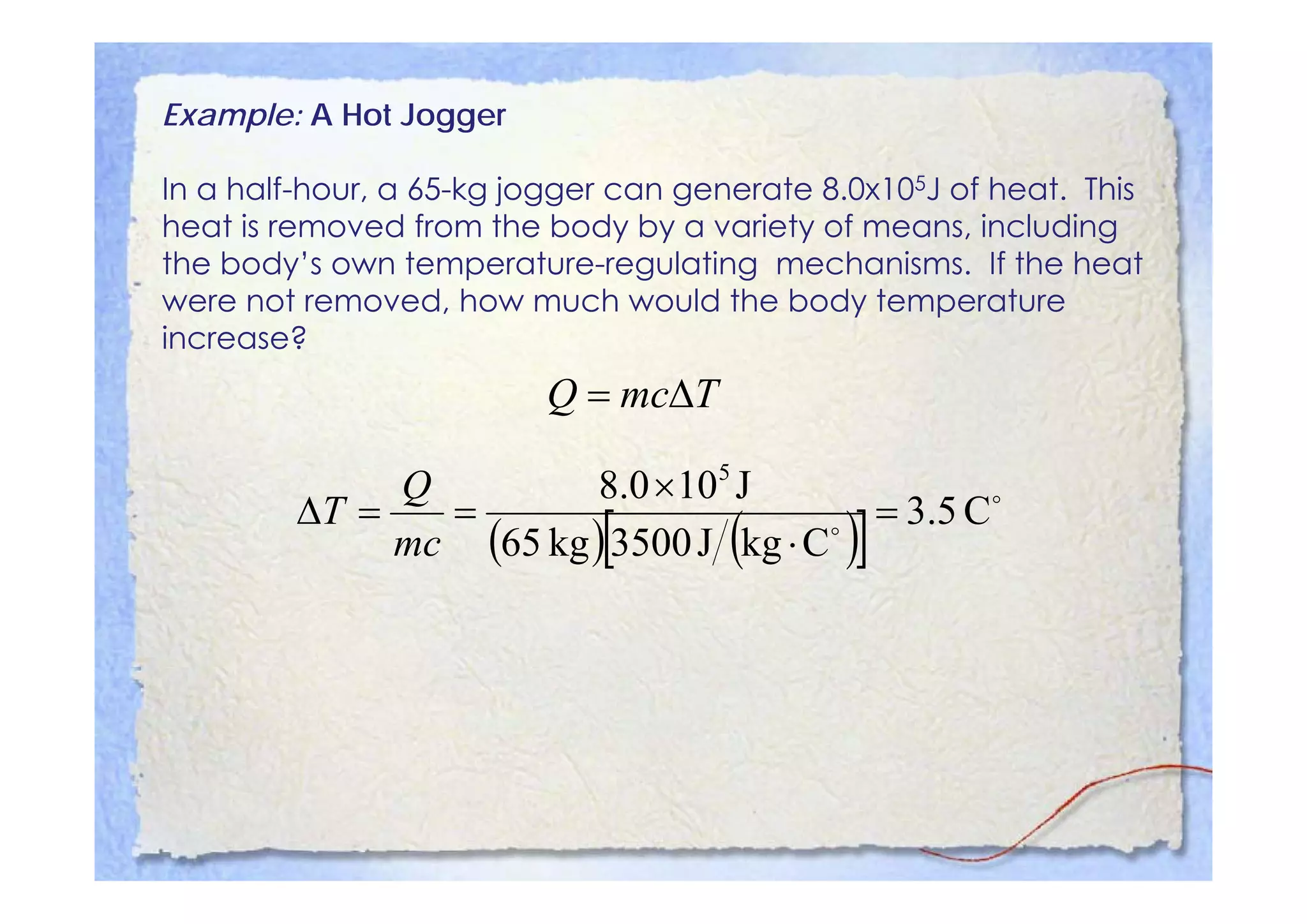
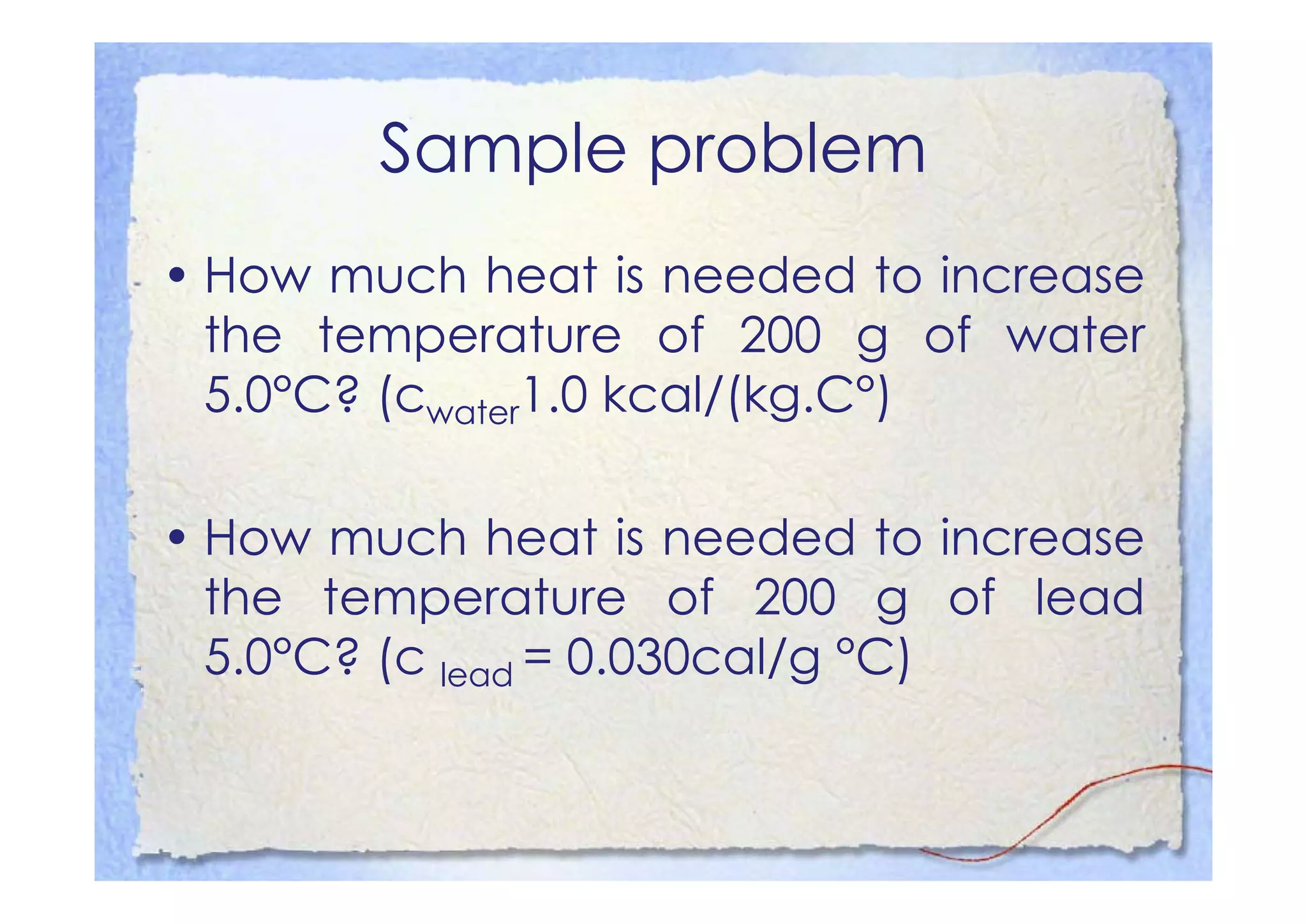







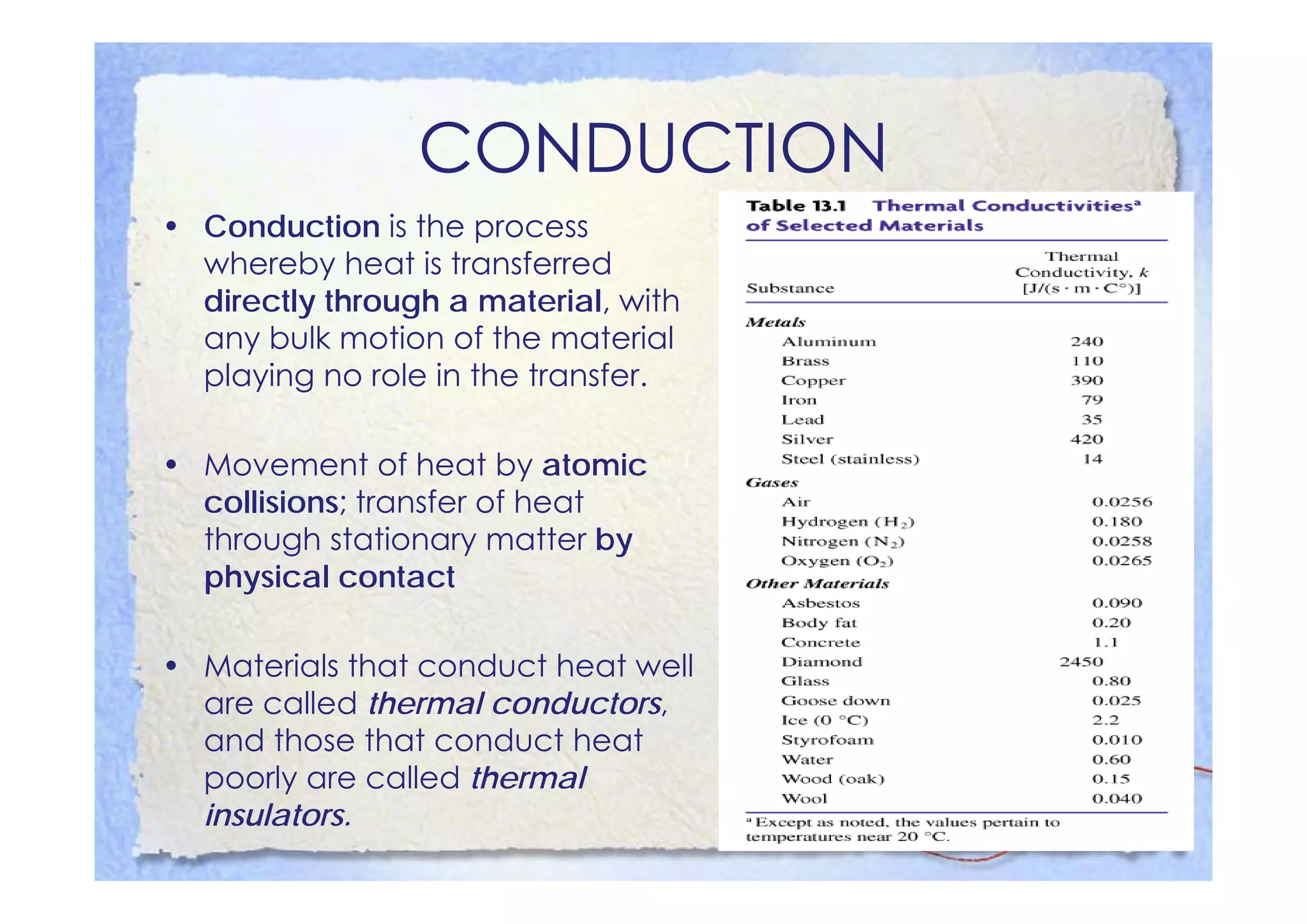

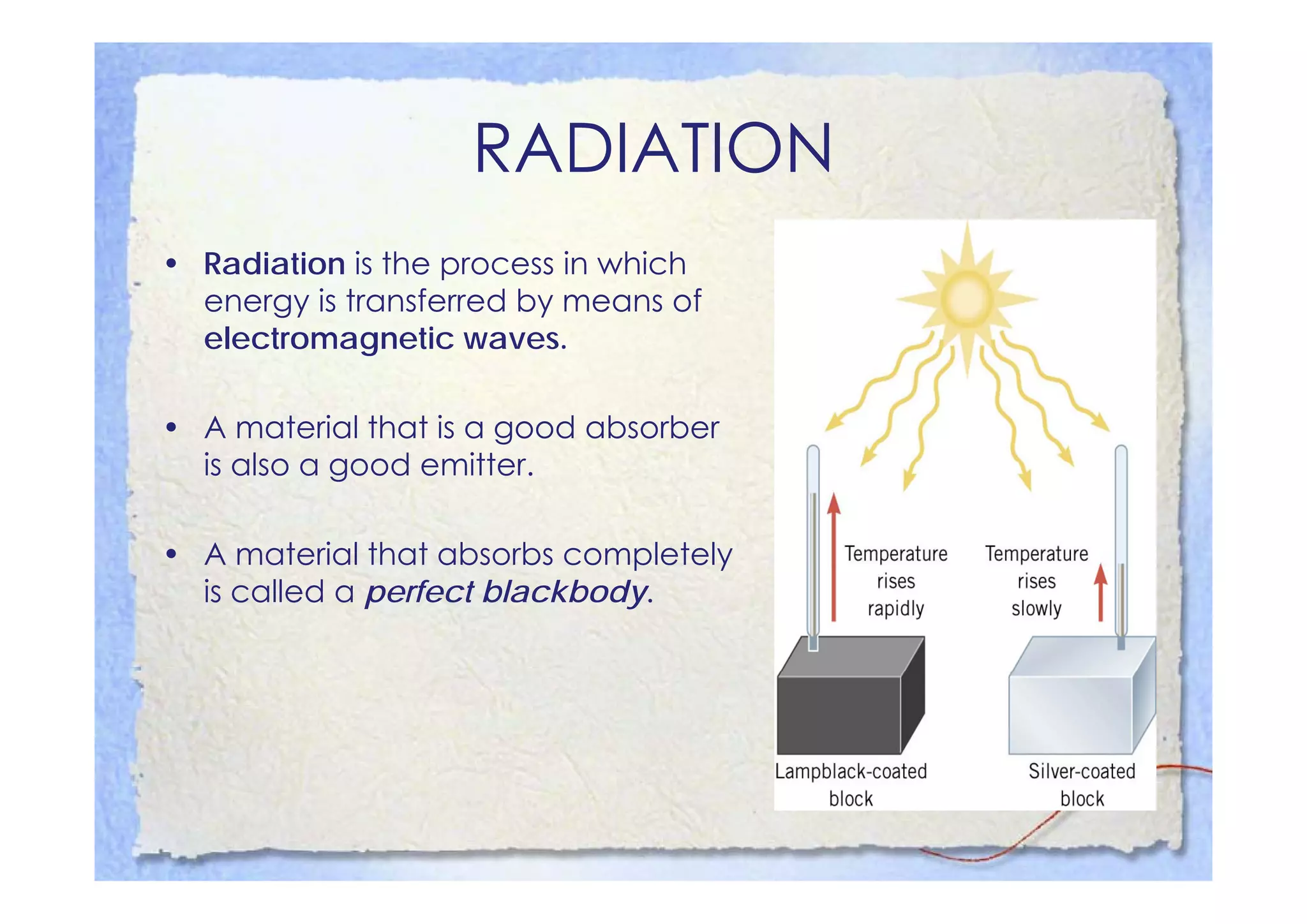






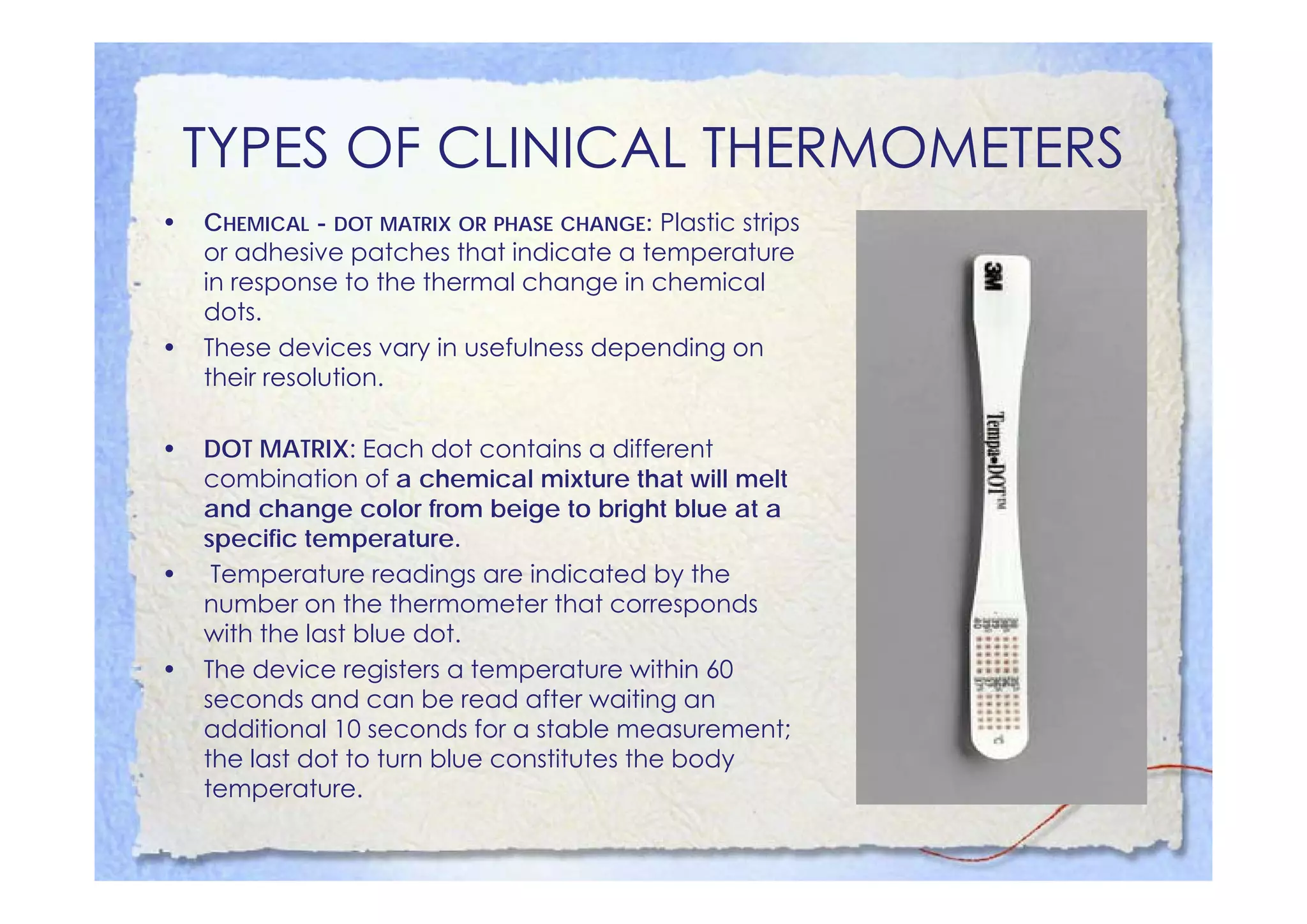

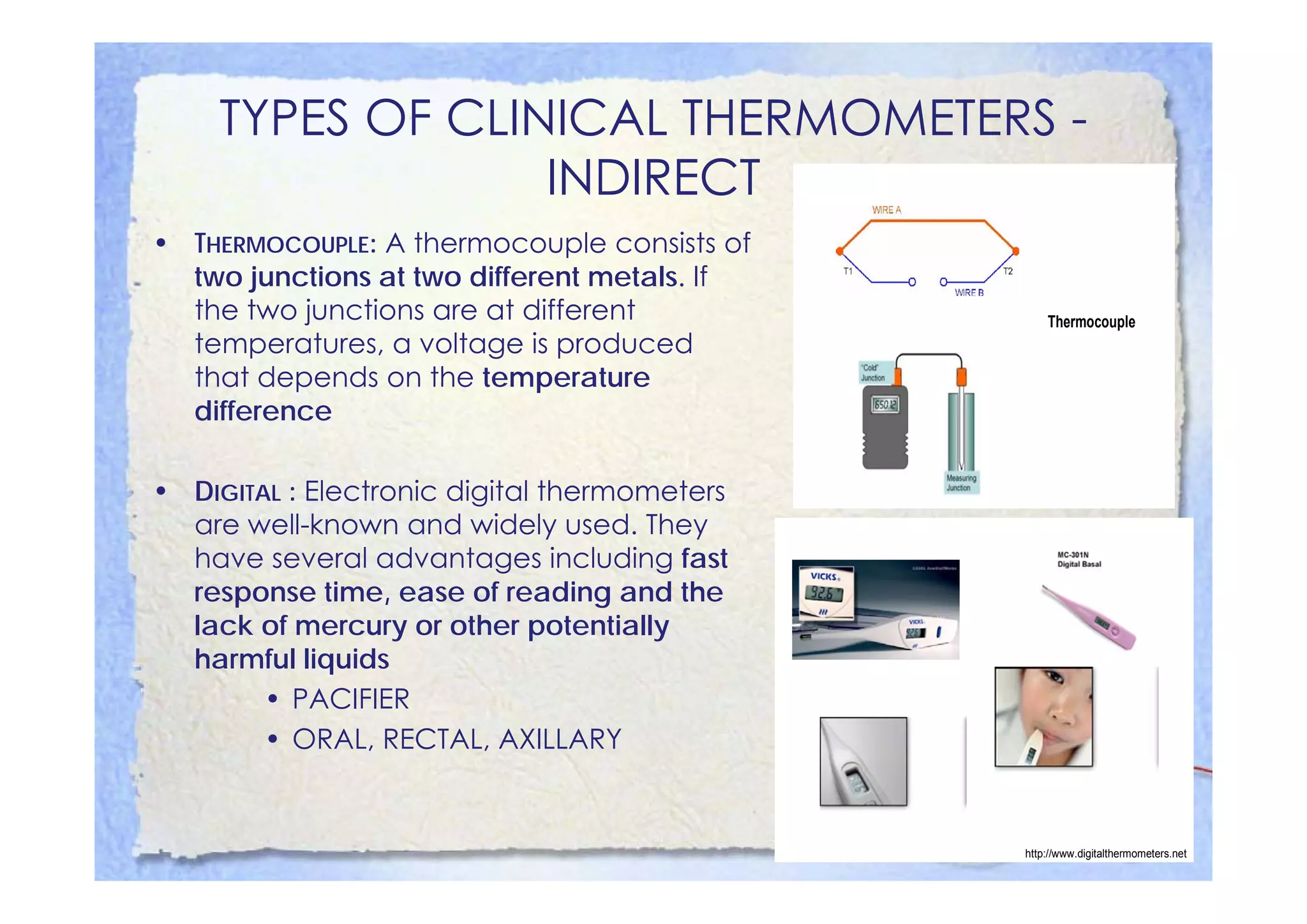

This document summarizes key concepts about temperature, heat transfer, and clinical thermometers. It defines common temperature scales (Celsius, Fahrenheit, Kelvin) and concepts like thermal expansion, heat, internal energy, specific heat capacity, phase changes, and latent heat. It describes different methods of heat transfer (conduction, convection, radiation). It outlines direct and indirect types of clinical thermometers, including liquid-in-glass, chemical dot matrix, digital, thermocouple, infrared thermometers and their uses.






























