This document provides an overview of key chemistry concepts including:
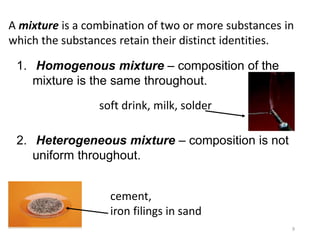
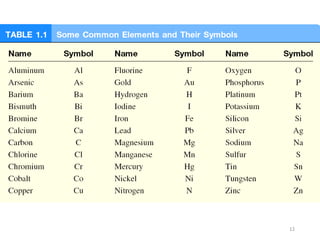
- Matter and its classifications including elements, compounds, and mixtures
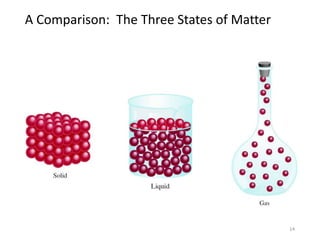
- The three states of matter and phase changes
- Physical and chemical properties of substances
- Energy, heat, and their relationship to chemical changes and physical processes
- Calorimetry and calculation of energy changes using specific heat