4.0 heat
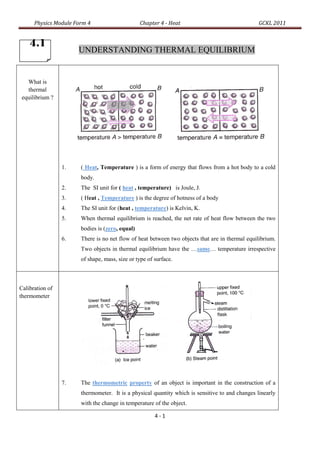
- 1. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium ? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat , temperature) is Joule, J. 3. ( Heat , Temperature ) is the degree of hotness of a body 4. The SI unit for (heat , temperature) is Kelvin, K. 5. When thermal equilibrium is reached, the net rate of heat flow between the two bodies is (zero, equal) 6. There is no net flow of heat between two objects that are in thermal equilibrium. Two objects in thermal equilibrium have the …same… temperature irrespective of shape, mass, size or type of surface. Calibration of thermometer 7. The thermometric property of an object is important in the construction of a thermometer. It is a physical quantity which is sensitive to and changes linearly with the change in temperature of the object. 4-1
- 2. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 8. Lower fixed point (l 0 )/ ice point : the temperature of pure melting ice/00C 9. Upper fixed point( l 100)/steam point: the temperature of steam from water that is boiling under standard atmospheric pressure /1000C 10. The lengths of the mercury column in the mercury-in-glass thermometer is 2.6 cm at 0OC and 22.6 cm at 100OC. When the thermometer is placed in hot water, the length of the mercury column is 16.9 cm. Calculate the temperature of the hot water. Liquid-in-glass 11. The liquid used in glass thermometer should thermometer (a) Be easily seen (b) Expand and contract rapidly over a wide range of temperature (c) Not stick to the glass wall of the capillary tube 12. List the characteristic of mercury (a) Opaque liquid (b) Does not stick to the glass (c) Expands uniformly when heated (d) Freezing point -390C (e) Boiling point 3570C 13. Which modification increases the sensitivity of the thermometer ? A. Increasing the size of the bulb B. Increasing the thickness of the bulb C. Reducing the diameter of the capillary tube D. Reducing the length of the capillary tube 4-2
- 3. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Check Yourself 1 1. The diagram below shows a thermometer is used to measure the temperature of hot water. When the thermometer and the hot water are in thermal equilibrium, which of the following is not correct ? 4. Diagram below shows the liquid levels from the bulb in three thermometers P, Q and R at certain temperatures. A. The temperature of hot water is equal to the temperature of the thermometer B. No heat flow between thermometer and hot water C. Heat flows from hot water to thermometer D. Heat flows from thermometer to hot water What is the temperature reading at thermometer R ? 2. Which of the following characteristics does a liquid-in-glass thermometer work ? A. 70.8 OC A. Volume of a fixed mass of liquid B. 65.4 OC B. Length of the liquid C. 62.5 OC C. Resistance of the liquid D. 57.7 OC D. Pressure of the liquid 3. Which of the following temperatures corresponds to zero on the Kelvin scale ? A. 273 OC B. 0 OC C. -273 OC D. 100 OC 4-3
- 4. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 4.2 4 UNDERSTANDING SPECIFIC HEAT CAPACITY Definition of Heat Capacity 1. Quantity of heat energy required to raise the temperature of an object by 10C. 2. The unit of heat capacity is J0C-1 3. Beaker A has (greater, same, less) heat capacity than beaker B. B A A A 4. This means that the (bigger, smaller) the mass, the (larger, smaller) the amount of heat stored. Definition of Specific Heat 1. Quantity of heat energy required to raise the temperature of 1 kg of a Capacity substance by 10C. 2. The unit of specific heat capacity is Jkg-1 0C-1 3. An object with low specific heat capacity can be heated up quickly, as it requires less heat to increase its temperature by 10C. It can be cooled quickly due to little amount of heat stored in it. 4. An object with high specific heat capacity takes longer time to heat up, as it requires more heat to increase its temperature by 10C. It is harder to cool down due to larger amount of heat stored in it. Q = Pt Pt = mc Q = Heat supplied P = Power of heater T = Time in seconds M = mass of substance C = Specific heat capacity = Increase in temperature 4-4
- 5. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Determining the heat capacity 1. The purpose of wrapping the aluminium blok with wool heat loss to or of aluminium block absorption of heat from the surrounding. 2. Oil in the holes for housing thermometer and the immersion heater is to improve the conduction of heat from the heater to the thermometer through the aluminium block. 3. The immersion heater of 50 W rated power is used for 5 minutes to heat up the aluminium block. If the mass of the alumimium block is 1.0 kg and the rise in temperature is 160C, what is the specific heat capacity of aluminium ? Pt 15000 C 937.5 Jkg-10C-1 m 1 16 4. Specific heat capacity calculated is usually larger than the standard value because some heat is lost to the surroundings. Aim To investigate the relationship between temperature rise and mass of water Experiment 800C 600C 800C 400C Hypothesis When the mass of water …increases… the temperature rise will …decrease…. Manipulated variable Mass of water Responding variable Rise in temperature Fixed variable Heating duration, water, weighing scale, power rating of heater used Apparatus Thermometer, water, beaker water, weighing scale Setup 4-5
- 6. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Procedure 1. 100 ml of water is placed in a 500 ml beaker. 2. A heater is placed in the water. 3. Heating process is carried out for 1minute. 4. Highest temperature achieved is recorded. 5. Step 2 to 4 is repeated for 200ml, 300ml, 400ml and 500ml of water. Analysis Volume of Final Initial Rise in 1/ (0C-1) water used, temperature, temperature, temperature, V (ml) T2 (0C) T1 (0C) = T2 – T1 (0C) 100 200 300 400 500 V Conclusion 1/ Applications of specific heat capacity Water as heating agent in heating radiator 4-6
- 7. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 2. Heat from hot water is released to the cooler surroundings of a room to achieve thermal equilibrium 3. Cold water will be recirculated to repeat the process continously 1. Cool water is pumped into the hot water reservoir to absorb a large amount of heat due to its high specific heat capacity. 4. Hot water is cooled by the air from the Water as a coolant in car cooling fins and the fan 2. High specific heat engine capacity of water allows it to absorb a large amount of heat from the engine 3. Cool water is recirculated through the engine blocks 1. Cool water is pumped and the process continues into the hot engine while the engine is running 3. Cool air 2. Hot air rises up blows from from the land the sea to replace the space left by 1. During the day the hot air Sea Breeze land gets hotter and than the sea convection because c land < c sea currents in (wind from the sea) the air are formed 3. Cooler air blows from the 2. hot air rises land to from the sea replace the space left Land Breeze (wind from the by the hot 1. During the land) air and night, the sea convection is hotter than currents in the land the air are because formed. c land < c sea 4-7
- 8. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Plastic handle High specific heat capacity Household apparatus and utensils Steel High specific heat capacity 3. Diagram below shows a bullet moving at a velocity of 60 ms-1 is embedded in a wooden Check Yourself 1 block. 1. Table below shows four types of liquid with their respective specific heat capacities and boiling points. All the liquids have the same mass and same temperatures of 30oC. If the same amount of heat is supplied to them, which liquid, A, B, C or D will boil first ? Liquid Specific heat Boiling point Assuming all the energy lost by the bullet is capacity (Jkg-10C-1) (oC) converted to heat energy and is absorbed by the bullet. What is the rise in temperature of A 3.0 50 the bullet ? ( Specific heat capacity of the B 5.0 80 bullet = 120 Jkg-10C-1) C 4.2 100 A. 0.5 oC D 0.2 200 B. 2.0 oC C. 30.0 oC 2. Table below shows the specific heat capacity D. 60.0 oC of four different metals. 4. Diagram below shows 200 g of water at 0 oC is poured into a cup containing 400 g of water at 80 oC . Assuming there is no heat loss to the surroundings. Which of the following is the most suitable metal to be used in a rice cooker for fast heating ? What is the final temperature of the mixture ? [ Specific heat capacity of water = 4200 Jkg-10C-1] A. P A. 53 oC B. Q C. R B. 60 oC D. S C. 66 oC D. 70 oC 4-8
- 9. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 5. Diagram below shows the temperature- time graph of two solids X and Y of equal mass but of different substances are heated simultaneously by identical heaters. Which of the following comparison is correct? A. Cx > Cy B. Cx < Cy C. Cx = Cy 4-9
- 10. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 4.3 4 UNDERSTANDING SPECIFIC LATENT HEAT Definition of 1. Latent Heat is the total energy absorbed or released when a substance changes its physical Latent Heat state completely at a constant temperature. 2. Latent Heat of fusion is heat absorbed when solid changes into liquid or heat released when liquid changes into solid at constant temperature. 3. Latent Heat of vaporization is heat absorbed when liquid changes into vapour or heat released when vapour changes into liquid at constant temperature. 4. Unit for latent heat is Joule (J). 5. Process in which solid directly changes into vapour is called sublimation. 1. Specific Latent Heat of fusion is heat absorbed when1 kgsolid changes into liquid or heat released when liquid changes into solid at constant temperature. Definition of Specific 2. Specific Latent Heat of vaporization is heat absorbed when1 kgliquid changes into vapour or Latent Heat heat released when vapour changes into liquid at constant temperature. 3. Unit for latent heat is Jkg-1. 4. When temperature remain constant, kinetic energy of the molecules remain constant. However energy absorbed is used to overcome forces of attraction and atmospheric pressure. 4 - 10
- 11. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Heating curve Fill the empty boxes for the heating curve below with the following words Solid, liquid, gas, boiling point, melting point, latent heat of fusion, latent heat of vaporization Calculation of specific latent heat of fusion Mass of water collected in Set A = 49.2 g Mass of water collected in Set B = 6.4 g Power of heater = 80 W Time interval of switching on the heater = 3 minutes 1. Calculate energy supplied by the heater. Q = Pt = (80)(3x60) = 14400 J 2. What is the mass of ice melted due to the heat absorbed from the surroundings?6.4 g 3. Determine the specific latent heat of fusion of ice. Pt (80)(3 60) 14400 L= = = = 3.364×105Jkg-1 m (49.2 6.4) 10 3 42.8 103 4 - 11
- 12. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 Check Yourself 1 1. Diagram below shows the cooling curve of a power of 1 kW. The beaker and its content is Gas. Temperatures T1 and T2 represents resting on an electronic balance which measures the mass of the beaker and its content. T1 T2 A. Solidification Room temperature When the water is boiling, it is found that 80 g of water is boiled away in 3 minutes. B. Boiling Point Solidification point What is the specific latent heat of vaporization C. MeltingPoint Freezing Point of water ? D. Melting Point Room temperature A. 2.10 x 106 Jkg-1 2. Diagram below shows the heating curve of a B. 2.15 x 106 Jkg-1 solid Y of mass 2 kg which is heated by a heater of 70 W power. Which statement C. 2.20 x 106 Jkg-1 below is NOT true ? D. 2.25 x 106 Jkg-1 4. Diagram below shows the arrangement of apparatus used to determine the specific latent heat of fusion of ice. There are two identical sets. One of the sets is called a control set which is without a power supply. A. Specific latent heat of Y is 10500 Jkg-1. B. Specific heat capacity of solid Y and liquid Y are the same C. Total heat used is 1120 J D. Melting point of Y is 10oC 3. Diagram below shows the arrangement of apparatus used to determine the specific latent heat of vaporization of water. The water in the The aim of the control set is beaker is heated by an immersion heater with a 4 - 12
- 13. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 A. To find the mass of ice melted due to the C. To detect any changes in the melting point heat absorbed from the surroundings of the ice B. To find the mass of water formed by D. To find the mass of water evaporated condensation from the vapour in the air 5. Heat produced in an engine block of car needs to be transferred out promptly to prevent overheating. This is done by circulating a suitable cooling liquid through the engine block. (a) What is meant by ‘specific heat capacity of water is 4200 Jkg-1oC-1 ? 4200 J of heat is required to raise the temperature of 1 kg of water by 1oC. (b) Based on the table above, (i) Explain the suitable characteristics of the cooling liquid to extract heat out of an engine block. High Specific Heat Capacity More heat can be extracted from the engine High specific latent heat of More heat can be extracted from the engine vaporization High boiling point Do not vaporize fast and cause unnecessary pressure to engine Low rusting rate Engine can last longer (ii) Decide which liquid is the most suitable and give reasons for your choice. Liquid B, high specific heat capacity, high specific latent heat of vaporization, high boiling point and low rusting rate. (c) Total energy released by an engine in 1 hour = 9.0 x 107 J Energy breakdown : mechanical 40% and heat 60% Mass of cooling liquid circulating in 1 hour = 150 kg Temperature of water entering the engine = 30oC Temperature of water exiting the engine = 60oC Based on the information above, (i) Calculate the power of the engine P = 9.0 x 107 J/3600 s = 2.5 x 107 W (ii) Calculate the amount of heat produced by the engine in one hour. Q = (60%)(9.0 x 107 J) = 5.4 x 107 J (iii) Calculate the specific heat capacity of the cooling liquid. Q = mc 5.4 x 107 J= 150(c)(60-30) c = 12,000 Jkg-1oC-1 4 - 13
- 14. Physics Module Form 4 Chapter 4 - Heat GCKL 2011 (d) Suggest two ways to dissipate the heat from the cooling liquid. 1. Use a cooling fan 2. Increase the surface area of the cooling coil 4 - 14