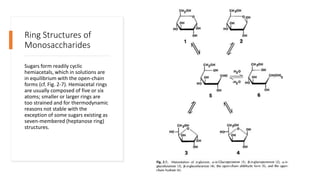
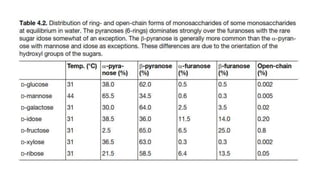
This document discusses cellulose. It provides three key points about cellulose:
1) Cellulose is a homopolysaccharide composed of β-D glucopyranose units linked together by (1–>4)-glycosidic bonds.
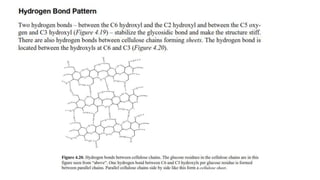
2) Cellulose molecules are linear and form intramolecular and intermolecular hydrogen bonds.
3) Cellulose consists of thousands of D-glucopyranosyl 1,4'-β-glucopyranosides as in cellobiose and forms large aggregate structures held together by hydrogen bonds. Cellulose is the main component of wood and plant fiber.