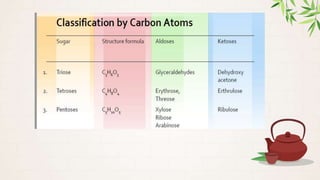
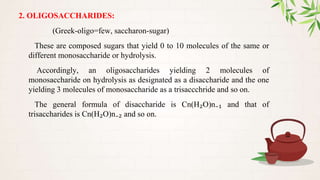
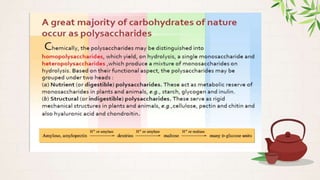
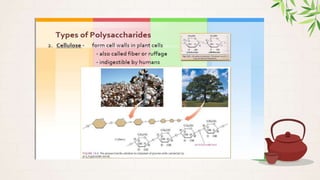
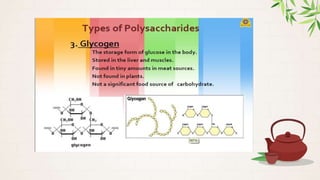
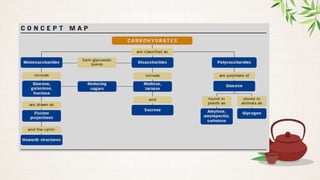
Carbohydrates are polyhydroxy aldehydes or ketones that are composed of carbon, hydrogen, and oxygen. They serve important functions in cells including as an energy source and structural component. Carbohydrates are classified as monosaccharides, oligosaccharides, or polysaccharides depending on their size. Starch and cellulose are two important polysaccharides - starch is used by plants for energy storage while cellulose provides structure in plant cell walls.