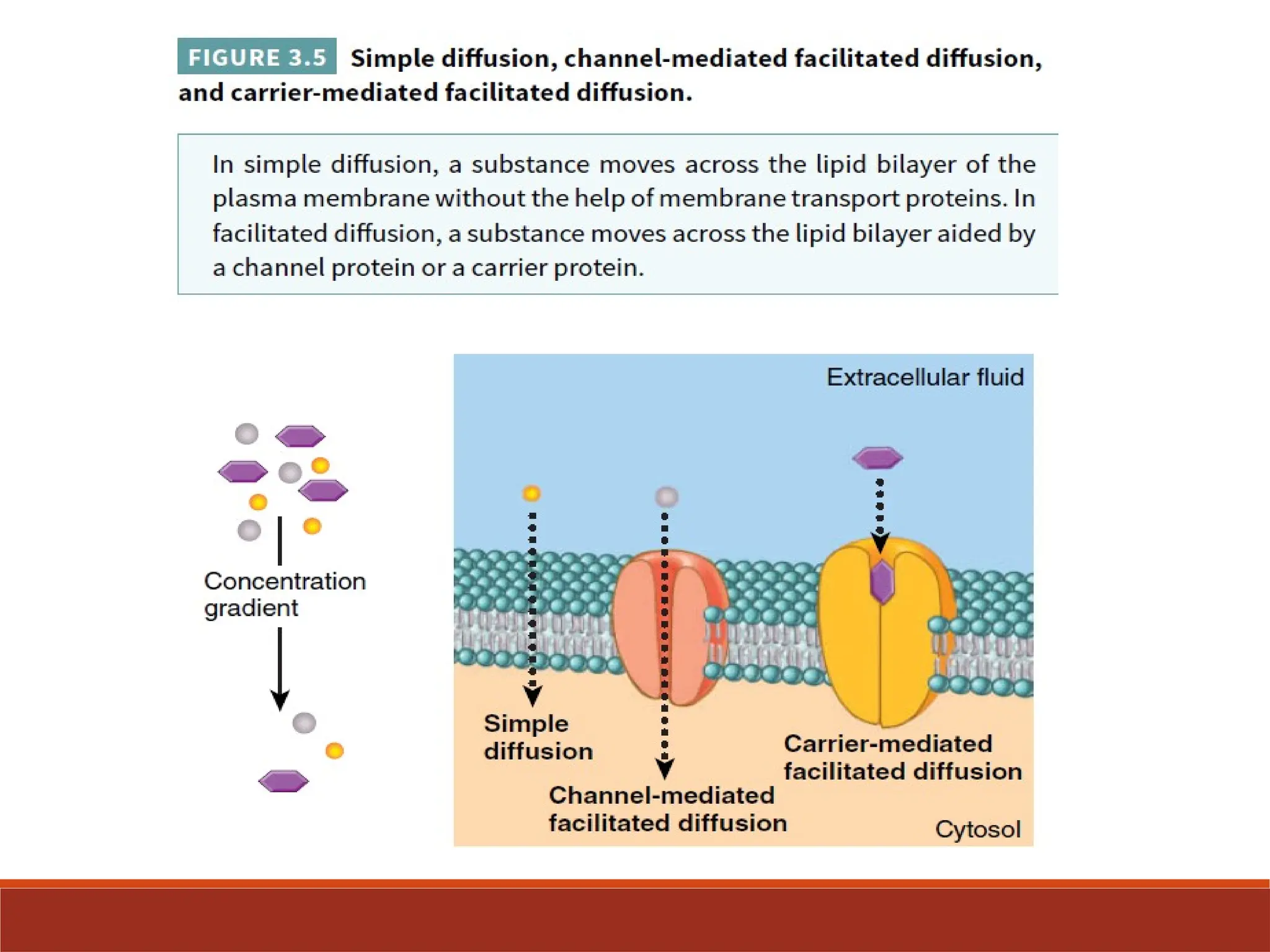
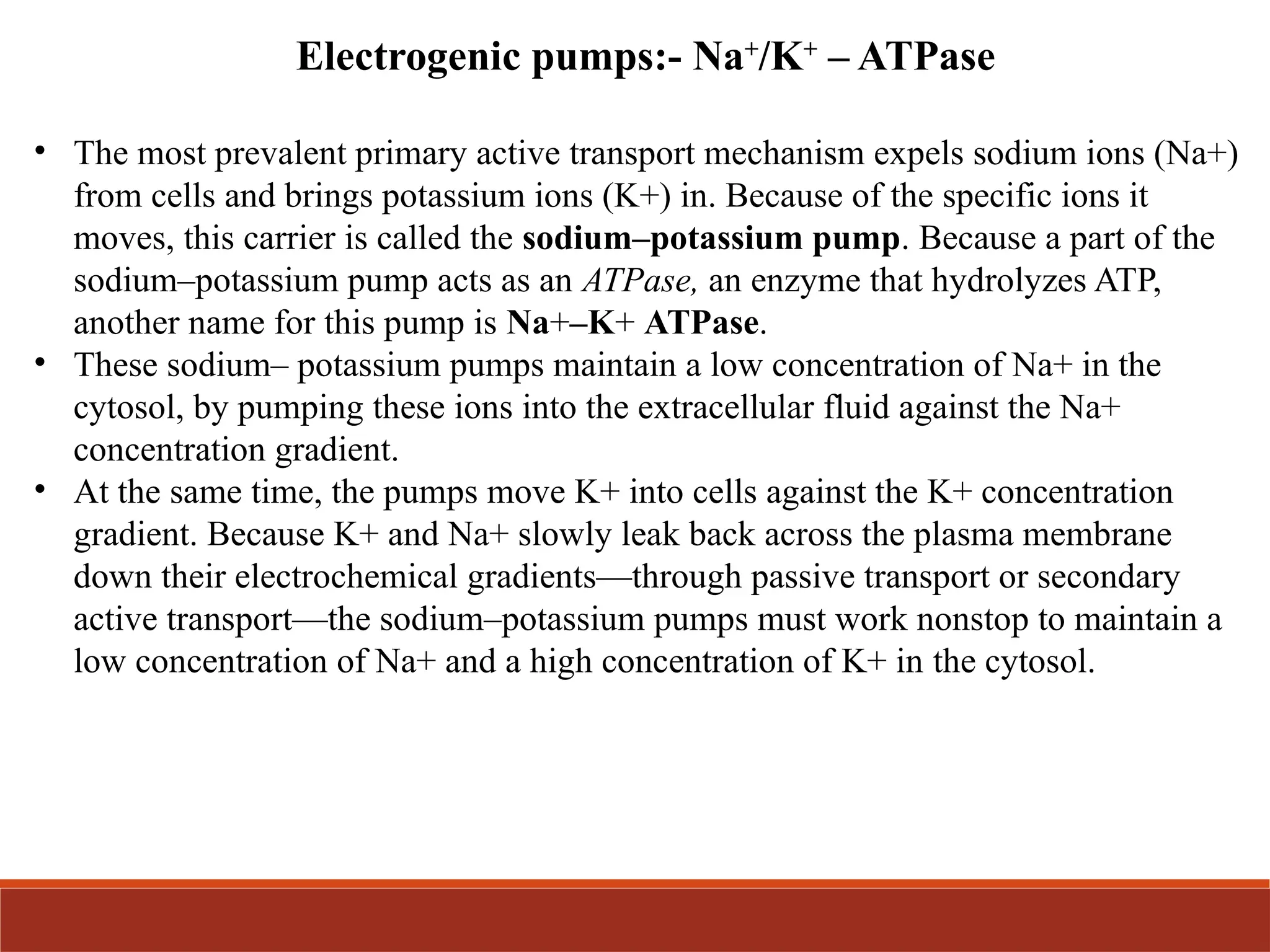
The document explains the transport mechanisms across the plasma membrane, highlighting its semi-permeable nature and the categories of transport which include passive transport, osmosis, active transport, and vesicular transport. It details different types of passive transport such as simple and facilitated diffusion, and explains active transport mechanisms driven by ATP, including primary and secondary active transport. The document also discusses the roles of carrier proteins and pumps in maintaining ion gradients essential for cellular functions.