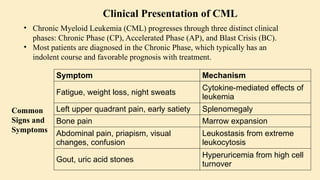
Chronic leukemias are slowly progressing cancers of the blood and bone marrow that involve mature or maturing hematopoietic cells. Unlike acute leukemia, which is aggressive and rapidly fatal without treatment, chronic leukemias progress over months or years and may be asymptomatic for a long time.
🔑 Key Features:
Slow onset, indolent course
Cells involved are more differentiated
Often detected incidentally during routine blood work
Includes:
Chronic Myeloid Leukemia (CML) – Myeloid origin
Chronic Lymphocytic Leukemia (CLL) – Lymphoid origin
Hairy cell leukemia
Prolymphocytic leukemia
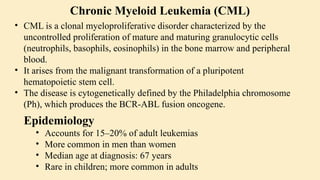
🔷 2. What is Chronic Myeloid Leukemia (CML)?
CML is a clonal stem cell disorder classified as a myeloproliferative neoplasm. It results in the uncontrolled proliferation of mature and maturing granulocytes (neutrophils, basophils, eosinophils) in the blood and bone marrow.
🔑 Key Characteristics:
Involves a pluripotent hematopoietic stem cell
Strongly associated with a genetic abnormality: the Philadelphia chromosome
Leads to bone marrow hyperplasia and leukocytosis
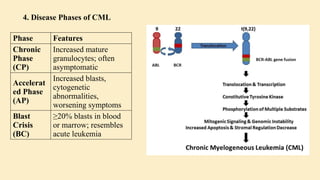
Progresses in phases:
Chronic phase
Accelerated phase
Blast crisis
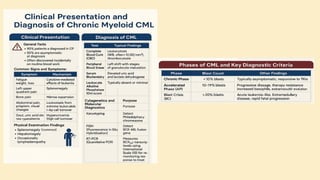
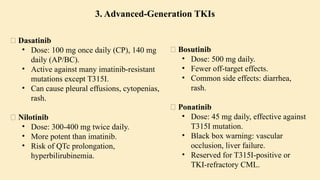
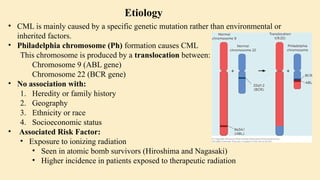
🔷 3. Etiology (Cause) of CML
CML is mainly caused by a specific genetic mutation rather than environmental or inherited factors.
✅ Known Facts:
Philadelphia chromosome (Ph) formation causes CML
This chromosome is produced by a translocation between:
Chromosome 9 (ABL gene)
Chromosome 22 (BCR gene)
❌ No association with:
Heredity or family history
Geography
Ethnicity or race
Socioeconomic status
⚠️ Associated Risk Factor:
Exposure to ionizing radiation
Seen in atomic bomb survivors (Hiroshima and Nagasaki)
Higher incidence in patients exposed to therapeutic radiation
🔷 4. Epidemiology of CML
📊 Statistics:
~8,990 new CML cases in the US (2019)
Accounts for 15–20% of adult leukemias
More common in men than women
👤 Patient Profile:
Median age at diagnosis: 67 years
Rare in children; more common in adults
🔷 5. Pathophysiology of CML
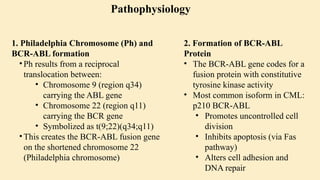
🔹 A. Philadelphia Chromosome (Ph)
Discovered in 1960
Caused by reciprocal translocation:
t(9;22)(q34;q11)
Creates the BCR-ABL fusion gene
🔹 B. BCR-ABL Fusion Gene
The fusion gene codes for a protein with tyrosine kinase activity
The most common abnormal protein: p210 BCR-ABL
Constitutively active (always “on”) → uncontrolled cell proliferation
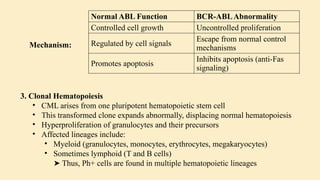
🧬 Mechanism:
Normal ABL Function BCR-ABL Abnormality
Controlled cell growth Uncontrolled proliferation
Regulated by cell signals Escape from normal control mechanisms
Promotes apoptosis Inhibits apoptosis (anti-Fas signaling)
🔹 C. Effect on Bone Marrow and Blood
Hyperproliferation of granulocytes and their precursors
Overcrowding leads to suppression of normal hematopoiesis
In later stages: cytopenias and marrow fibrosis
🔹 D. Clonality and Pluripotent Origin
Disease arises from one mutated stem cell
Affects myeloid and lymphoid progenitors (seen in early progenitor cells)
Leads to monoclonal expansion of malignant
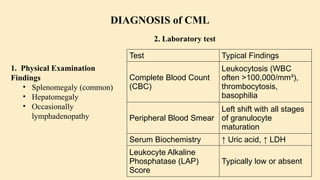
![Parameter Finding
Cellularity
Markedly
hypercellular (75–
90%)
Myeloid:
Erythroid Ratio
Increased (10–
30:1)
Blasts <10% in CP
Megakaryocytes
Increased but
normal
morphology
Fibrosis
Typically absent
or minimal
Test Purpose
Karyotyping
Detect Philadelphia
chromosome (t[9;22]
[q34;q11])
FISH
(Fluorescence
In Situ
Hybridization)
Detect BCR-ABL
fusion gene
RT-PCR
(Quantitative
PCR)
Measure BCR-ABL1
transcript levels
using International
Scale (IS) for
monitoring response
to treatment
3. Bone Marrow Biopsy 4. Cytogenetics and Molecular Diagnostics](https://image.slidesharecdn.com/chronicleukemia-250713170042-4b8fbffe/85/Leukemia-CHRONIC-LEUKEMIA-CML-CLL-pptx-11-320.jpg)