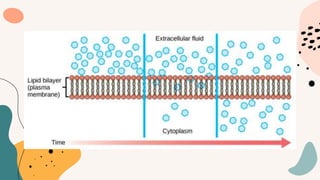
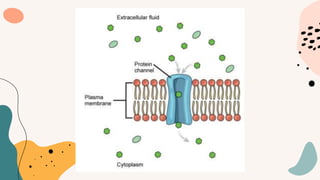
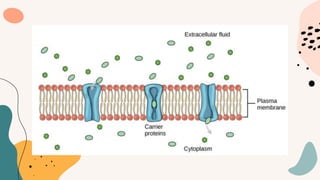
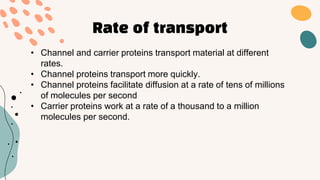
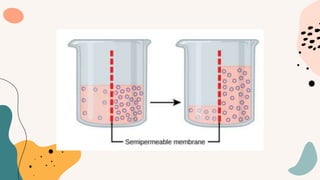
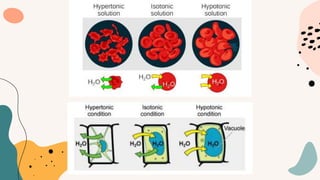
This document discusses cell membrane transport mechanisms. It begins by explaining that plasma membranes are selectively permeable, allowing some substances to pass through freely via diffusion while requiring special transport proteins for others. It then examines different transport mechanisms in detail. These include passive transport processes like diffusion and facilitated transport via channel or carrier proteins, as well as active transport processes. Osmosis is discussed as a type of diffusion dependent on a water concentration gradient. The concepts of tonicity, osmolarity, and their effects on cell volume are also covered. The document seeks to explain the key functions and factors involved in transport across the cell membrane.