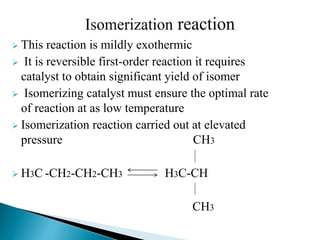
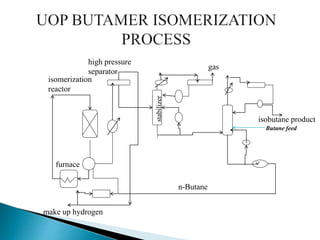
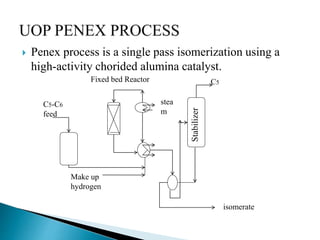
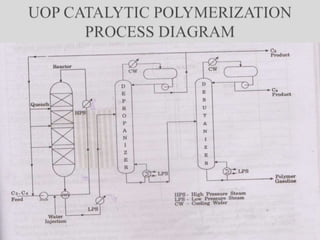
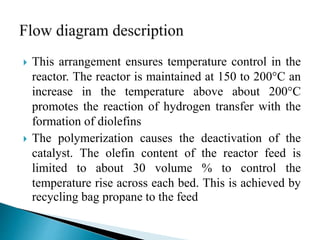
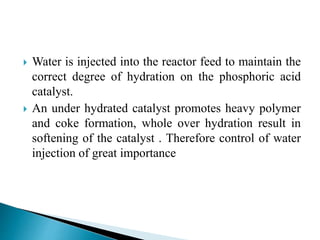
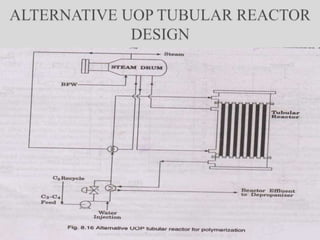
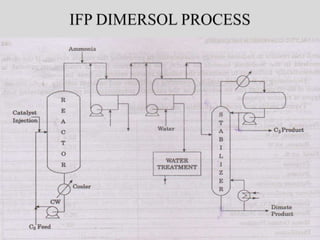
The document discusses various petroleum refining processes including catalytic isomerization, UOP Butamer and Penex isomerization processes, catalytic polymerization, UOP catalytic polymerization process, alternative UOP tubular reactor design, and the IFP Dimersol process. It provides details on the chemistry and operating principles of each process, including feedstocks, reactions, yields, equipment used and product properties. The overall purpose is to describe several key technologies used in refineries to convert petroleum fractions into higher octane products like gasoline.