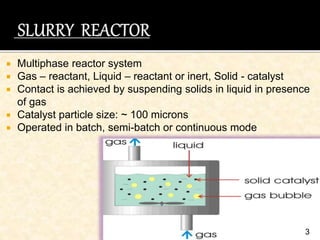
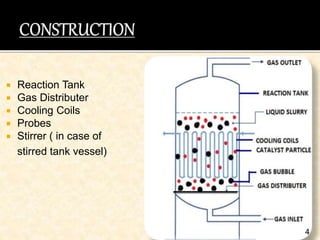
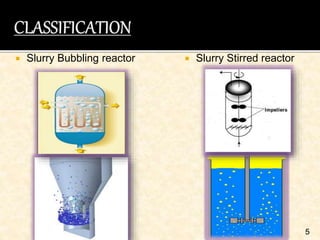
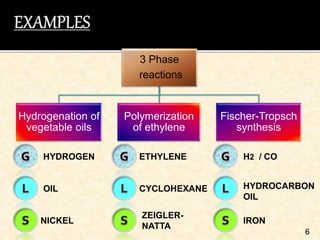
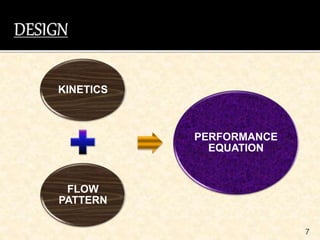
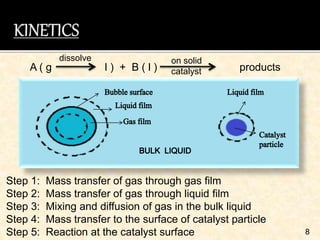
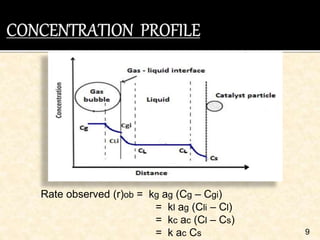
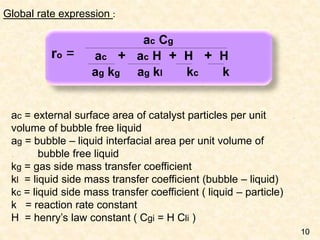
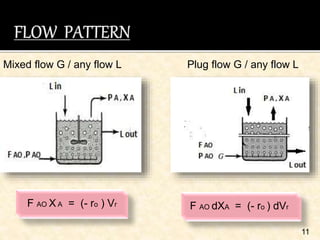
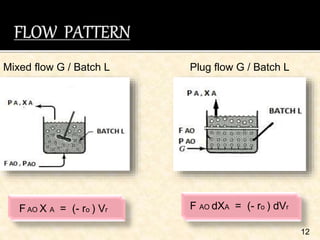
This document discusses multiphase reactors, which involve gas, liquid, and solid phases. It covers the construction, classification, examples, design considerations, kinetics, advantages, and applications of these reactors. Specifically, it examines slurry bubble column reactors and slurry stirred tank reactors. It provides examples of industrial processes using multiphase reactors like hydrogenation, polymerization, and Fischer-Tropsch synthesis. Rate equations are also presented to model reactions in these complex systems.