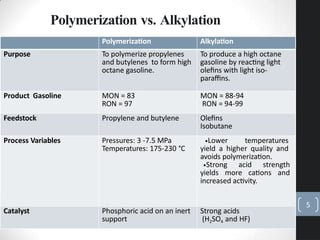
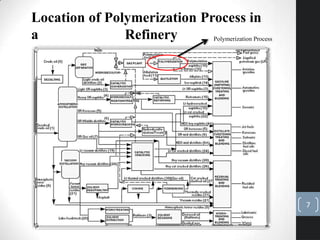
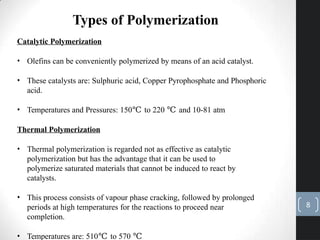
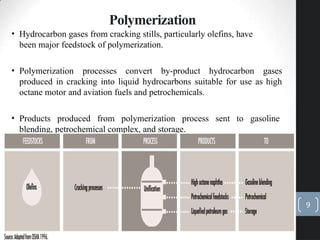
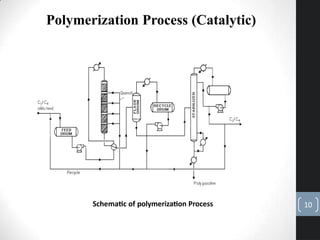
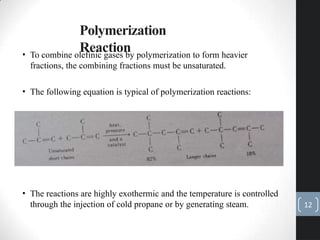
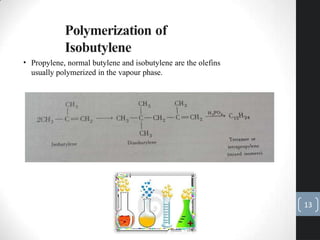
The document discusses the polymerization process in the petroleum industry, specifically how light olefins are converted into higher molecular weight hydrocarbons suitable for gasoline. It outlines the historical development of polymerization alongside alkylation, comparing their methods, purpose, and products. The document also covers the operational aspects, safety considerations, and health implications associated with polymerization in refinery settings.