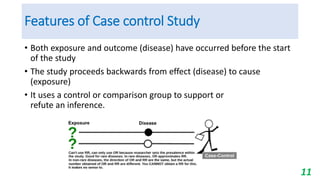
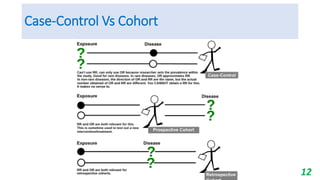
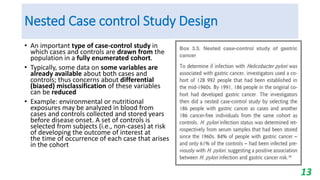
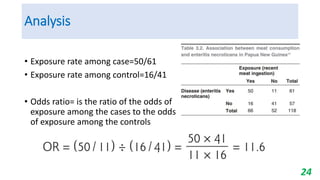
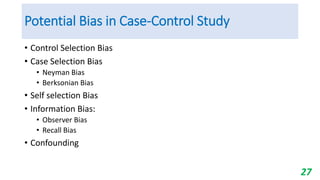
This document provides an overview of case-control studies, including their key features, steps, and potential biases. Case-control studies compare exposures in individuals with an outcome (cases) to those without the outcome (controls) to identify potential risk factors. Steps include selecting cases and controls, measuring exposures through questionnaires/interviews, and analyzing data to estimate disease risk associated with exposures. Potential biases include selection, information, and confounding biases. Case-control studies are useful for rare diseases and identifying multiple risk factors, though they only estimate relative risk.