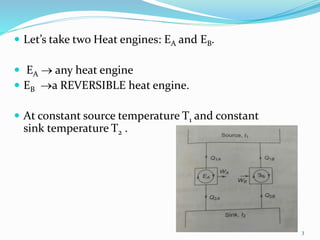
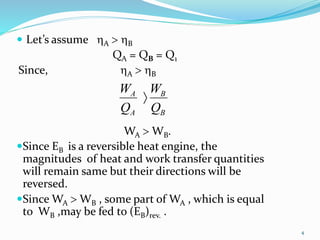
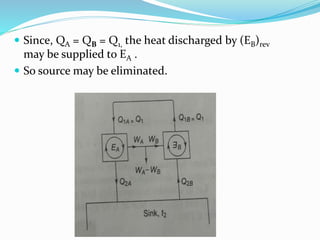
1) Carnot's theorem states that no heat engine operating between a constant temperature heat source and sink can be more efficient than a reversible heat engine.
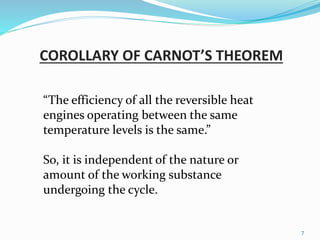
2) This leads to the corollary that the efficiency of all reversible heat engines operating between the same temperature levels is the same, regardless of the working substance.
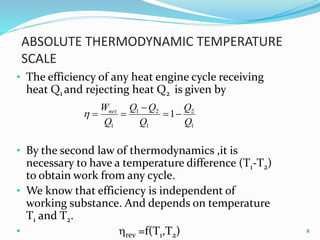
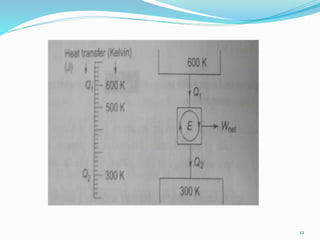
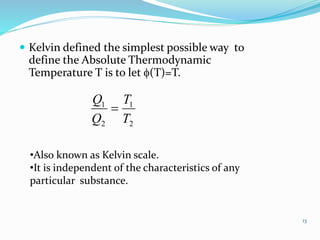
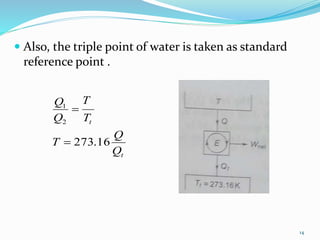
3) An absolute thermodynamic temperature scale can be defined based on this, with the efficiency of any heat engine cycle depending only on the temperature difference between the heat source and sink. Kelvin defined the simplest scale where the temperature is directly proportional to the efficiency.