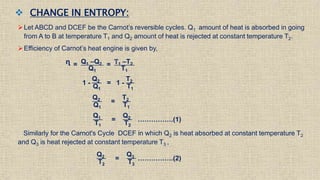
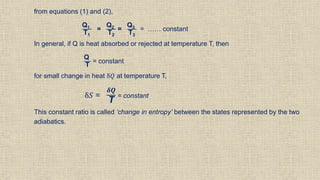
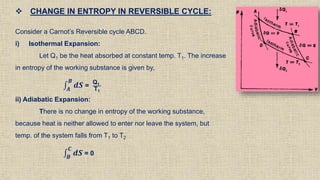
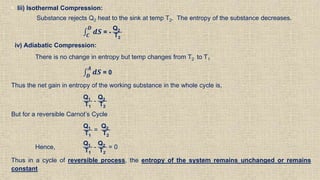
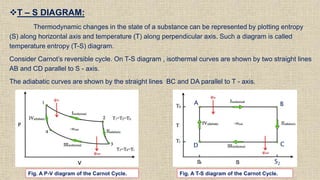
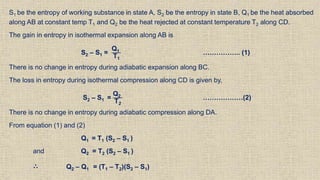
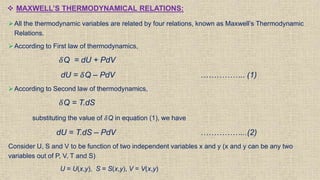
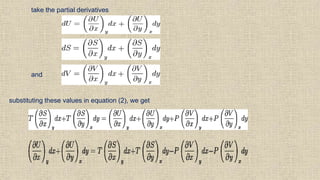
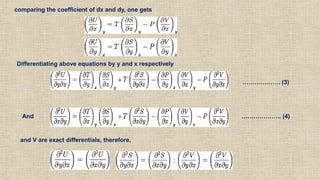
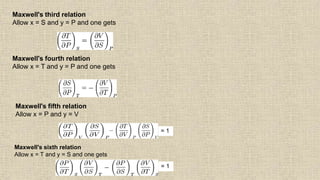
The document provides an overview of thermodynamics, focusing on concepts such as entropy, reversible cycles, and the laws governing thermodynamic processes. It explains the principles of entropy, its relationship to heat and temperature, and the implications of the second and third laws of thermodynamics, including the idea of heat death of the universe. Additionally, it details Maxwell’s thermodynamic relations, which establish connections between various thermodynamic variables.