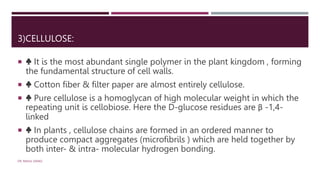
The document discusses carbohydrates as polyhydroxy aldehydes, ketones, or their derivatives, classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides based on their size and structure. It details their functions as energy sources, food reserves, and structural components in animals and plants, along with specific examples and their roles in various biological processes. It emphasizes the importance of carbohydrates in nutrition and their classification into soluble and insoluble types depending on digestibility.




![POLYSACCHARIDES
– Homoglycans
I. • Pentosans (C5H8O4)n, for example, arabans and xylans
II. • Hexosans (C6H12O6)n, for example, starch, cellulose, mannans, levans, and glycogen
– Heteroglycans Hemicelluloses, pectins, exudate gums, seaweed polysaccharides (algin, carrageenans,
agar, aminopolysaccharides [e.g., chondroitin and hyaluronic acid], and sulfated polysaccharides [e.g.,
chondroitin sulfate])
• Conjugated carbohydrates
i. – Glycolipids: Glyceroglycolipids and sphingolipids
ii. – Glycoproteins : Mucins, immunoglobulins, and membrane-bound hormone receptors
DR. RAHUL DANGI](https://image.slidesharecdn.com/presentationcarbohydrates-221221205645-6374c533/85/Presentation-carbohydrates-classification-pptx-7-320.jpg)













![1)Starch :
• It is present in many plants as a reserve carbohydrate . It is most abundant in seeds , fruits , tubers & roots . • Starches
naturally occur in the form of granules . • Starches differ in their chemical composition, except in rare instances, mixtures of
structurally different polysaccharides, amylose (straight chain polymer) & amylopectin (branched chain polymer). • The ratio of
amylose to amylopectin varies with the sources of starch, but it is usually 1:3 (g/g) • Amylose is mainly long linear in structure,
the α-D-glucose residues being linked between carbon 1 atom of one molecule & carbon atom 4 of the adjacent molecule. It
has no branch 38 1)Starch : • Amylopectin has a small chain brush like structure containing primarily α-(1→4) linkages in the
main chain and an appreciable number of α(1→6) linkages in branch chain. – Starch is made of repeating units of the
disaccharide MALTOSE – so it is really all: GLUCOSE • Starch granule are insoluble in cold water , but when a suspension in water
is heated, the granules swell & eventually gelatinise. • Animals consume large quantities of starch in cereal grains, cereal
byproducts and tubers.
• On the basis of the rates of their in vitro enzymatic digestion, there are three types of starch: • (1) rapidly digested starch (with
high amylopectin content and high digestibility containing 100% amylopectin and 0% amylose), freshly cooked starch, and
white bread; • (2) slowly digested starch (with a certain weight ratio of amylose to amylopectin of, e.g., 45:55 [more amylopectin
than amylose] and low digestibility), such as starch in most raw cereals (dent corn varieties, barley, wheat, and rice), whose
semicrystalline structure renders it less accessible to digestive enzymes; and • (3) resistant starch (with high-amylose content
and limited digestibility), such as high-amylose corn, high-amylose wheat, legumes, and bananas
• In its natural state, tuber and grain starch (e.g., in potatoes) exist in a water-insoluble granular form, which resists digestion in
the small intestine of nonruminants . Such starch-containing foods must be cooked before they can be more effciently utilized
by chickens and pigs. • The cooking of foods markedly aids in their digestion by animals through breaking down (gelatinizing)
and solubilizing starch granules.
DR. RAHUL DANGI](https://image.slidesharecdn.com/presentationcarbohydrates-221221205645-6374c533/85/Presentation-carbohydrates-classification-pptx-21-320.jpg)