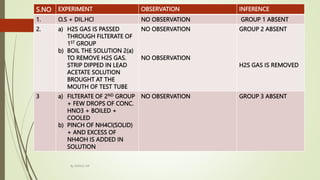
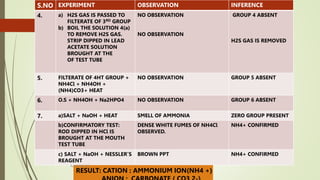
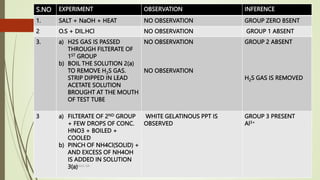
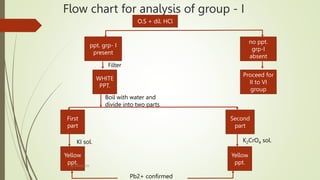
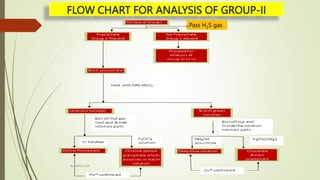
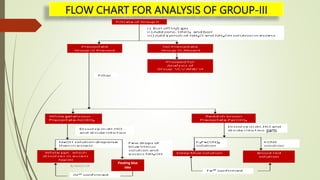
The document provides information on qualitative analysis to identify cations and anions in salts. It discusses the different groups of cations like group 1, 2, 3 etc. and different tests to identify them like using H2S gas, ammonium hydroxide, sodium hydroxide etc. It also explains the process to prepare original solution and soda extract for identifying cations and anions respectively. Flow charts are given for analytical procedures to identify specific cations like group 1, 2, 3 etc. Confirmatory tests to identify common anions like carbonate, chloride, sulfate etc. are also outlined. Two sample experiments performed to identify the cation and anion in two unknown salts are described at the end.








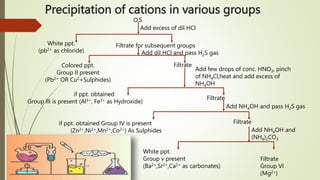
![1
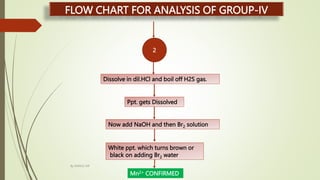
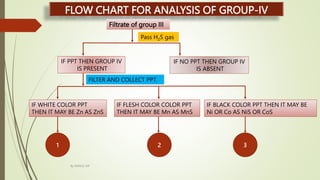
Dissolve in dil.HCl and boil off H2S gas. Then divide it into two parts
With first part add NaOH
solution dropwise
With first part add K4[Fe(CN)6]
White ppt. soluble in
excess NaOH
Bluish White ppt.
FLOW CHART FOR ANALYSIS OF GROUP-IV
Zn2+ CONFIRMED
By MANOJ SIR](https://image.slidesharecdn.com/chemistrypractical-230111145359-911e5418/85/CHEMISTRY-PRACTICAL-pptx-14-320.jpg)