Embed presentation
Downloaded 136 times



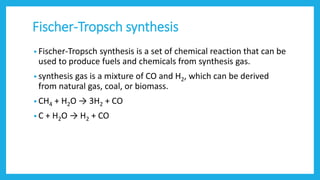

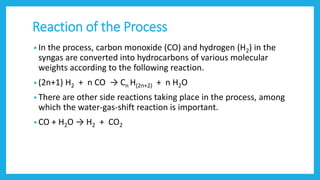
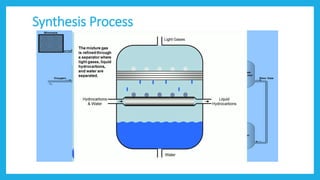



The document discusses Fischer-Tropsch synthesis, which produces liquid fuels and chemicals from synthesis gas. Synthesis gas is a mixture of carbon monoxide and hydrogen that can be derived from natural gas, coal, or biomass. The process was invented in the 1920s by German inventors Franz Fischer and Hans Tropsch. In the process, carbon monoxide and hydrogen in the syngas react to form hydrocarbons of various molecular weights, with water as a byproduct. The liquid fuels produced can be used for vehicles, power generation, cooking, and as raw materials for various industries.








