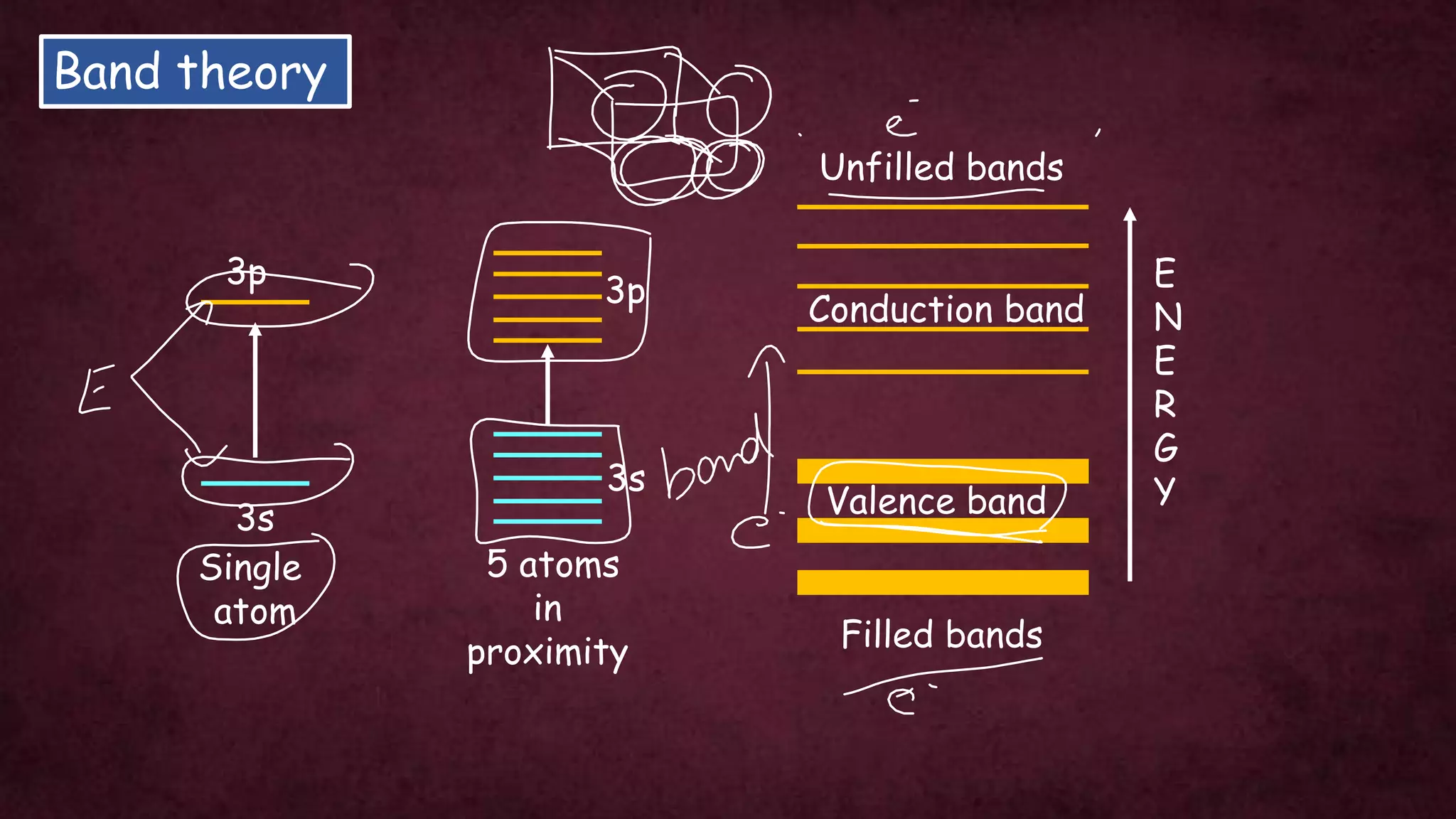
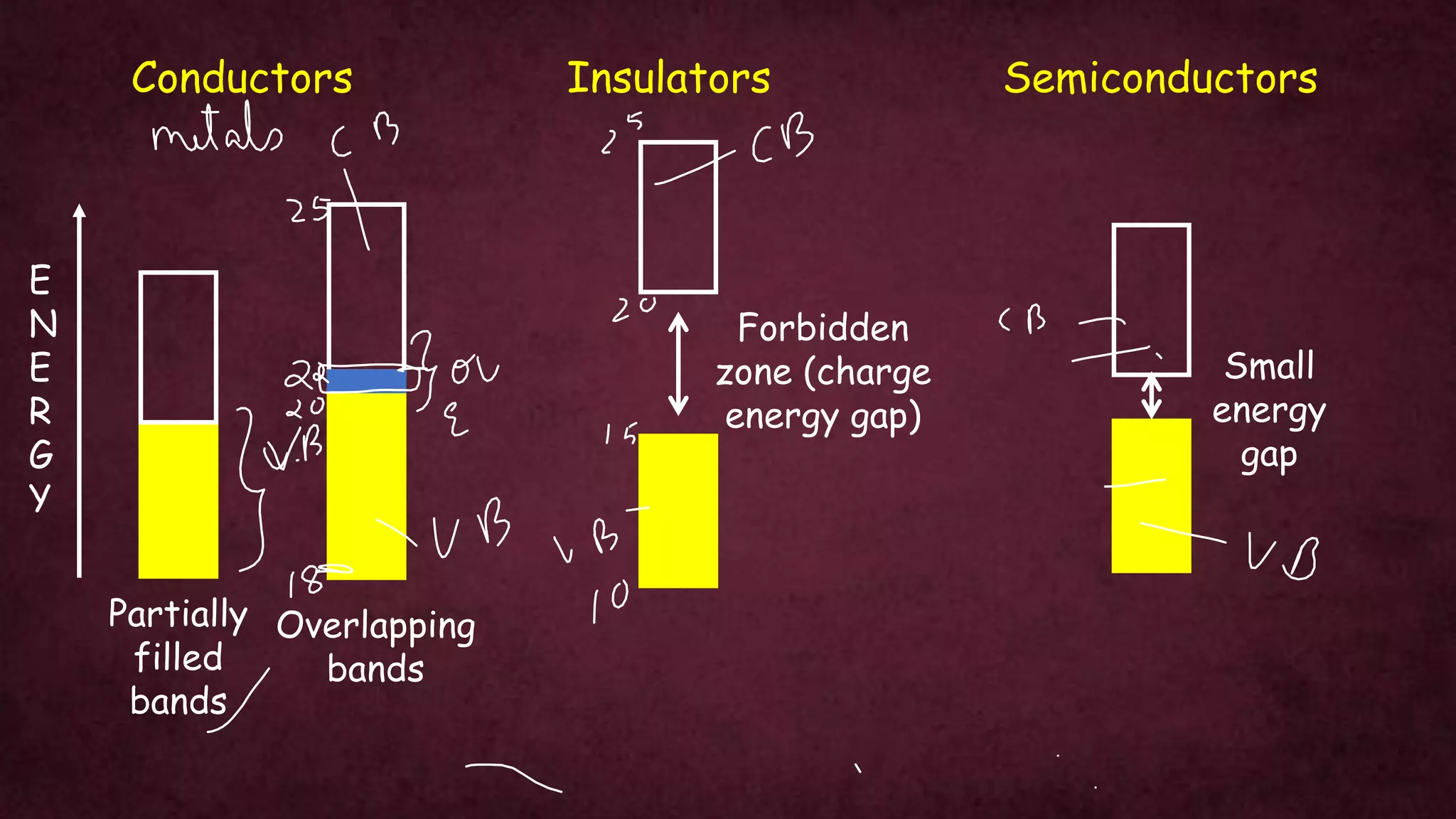
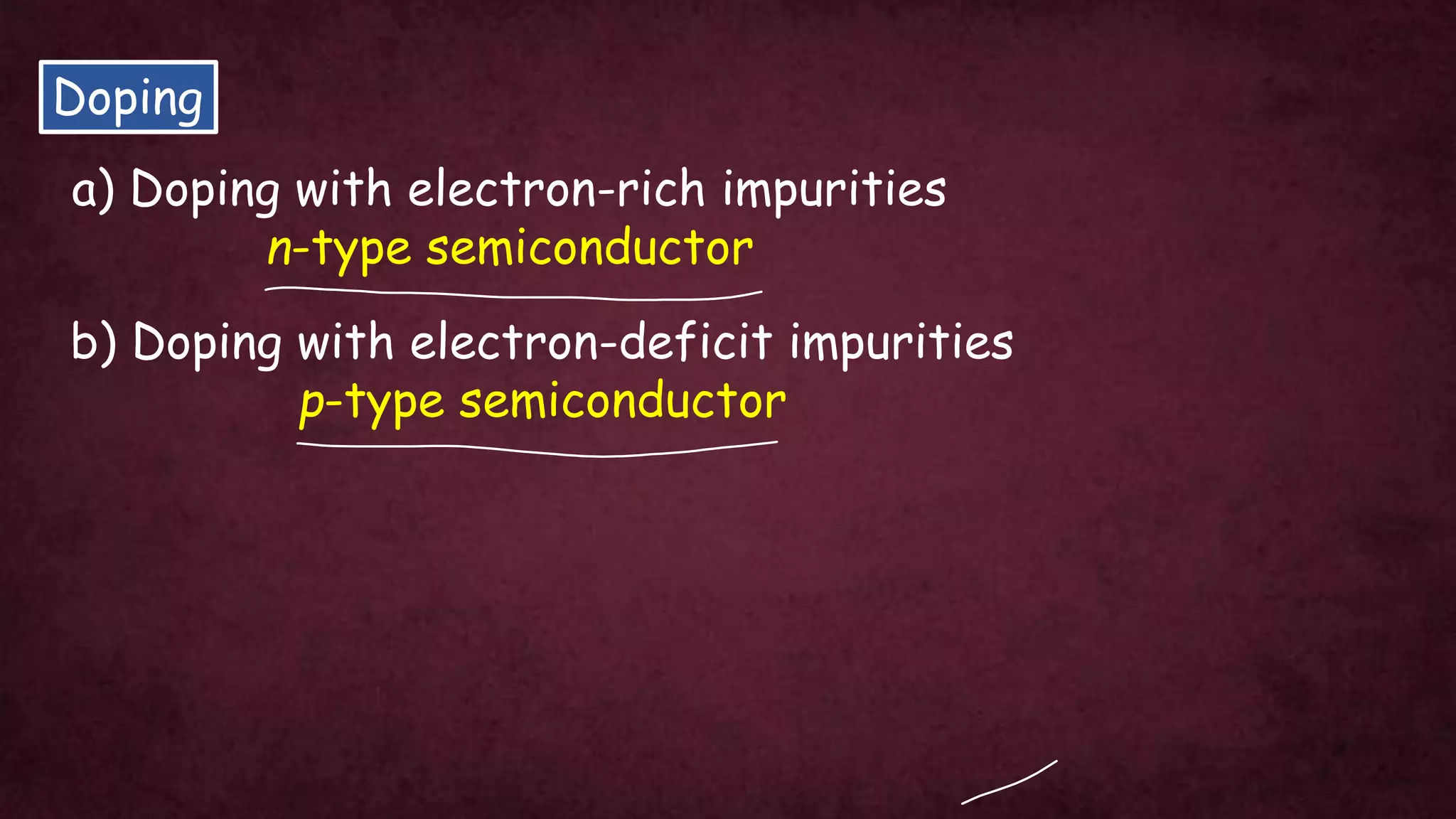
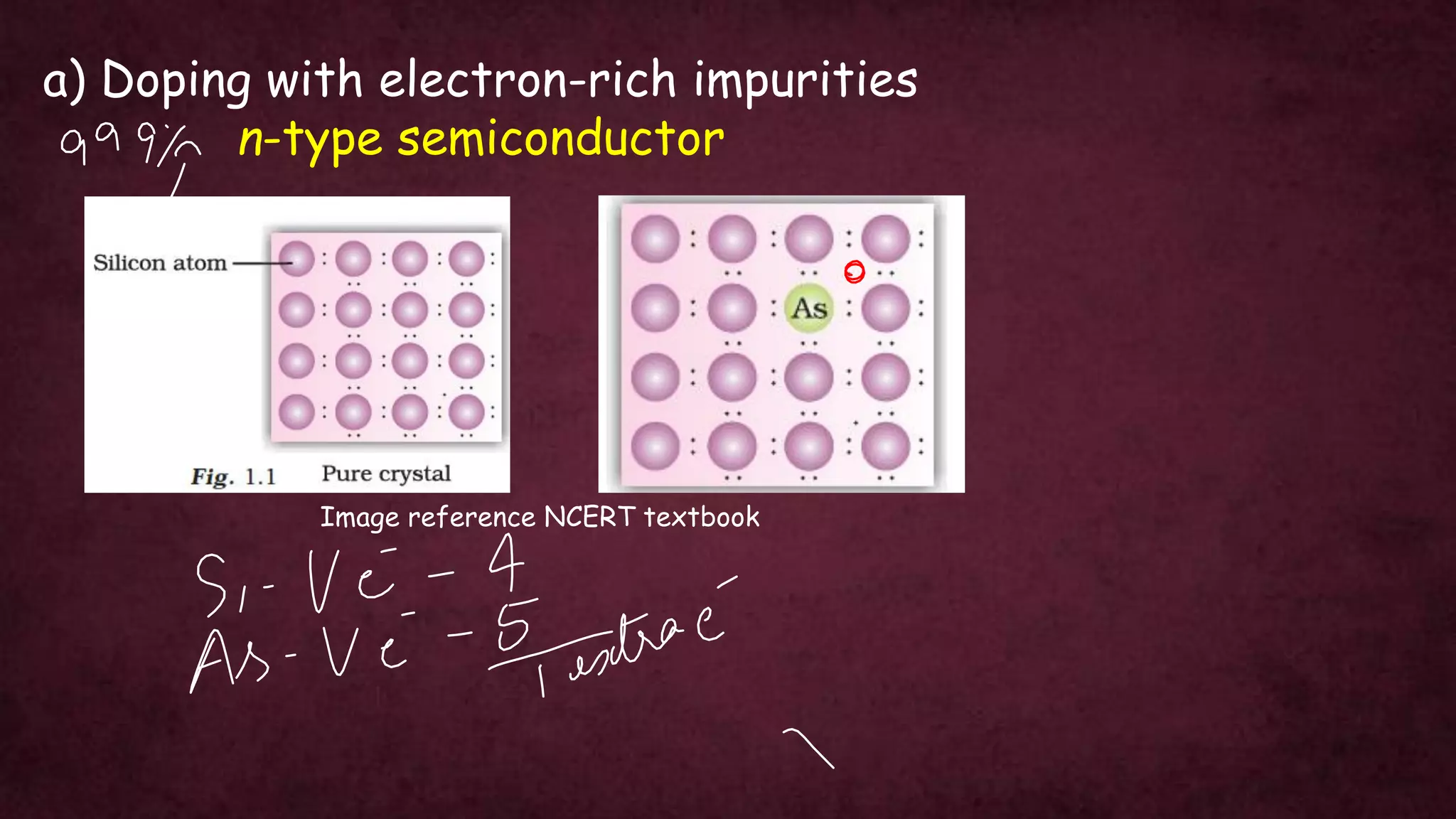
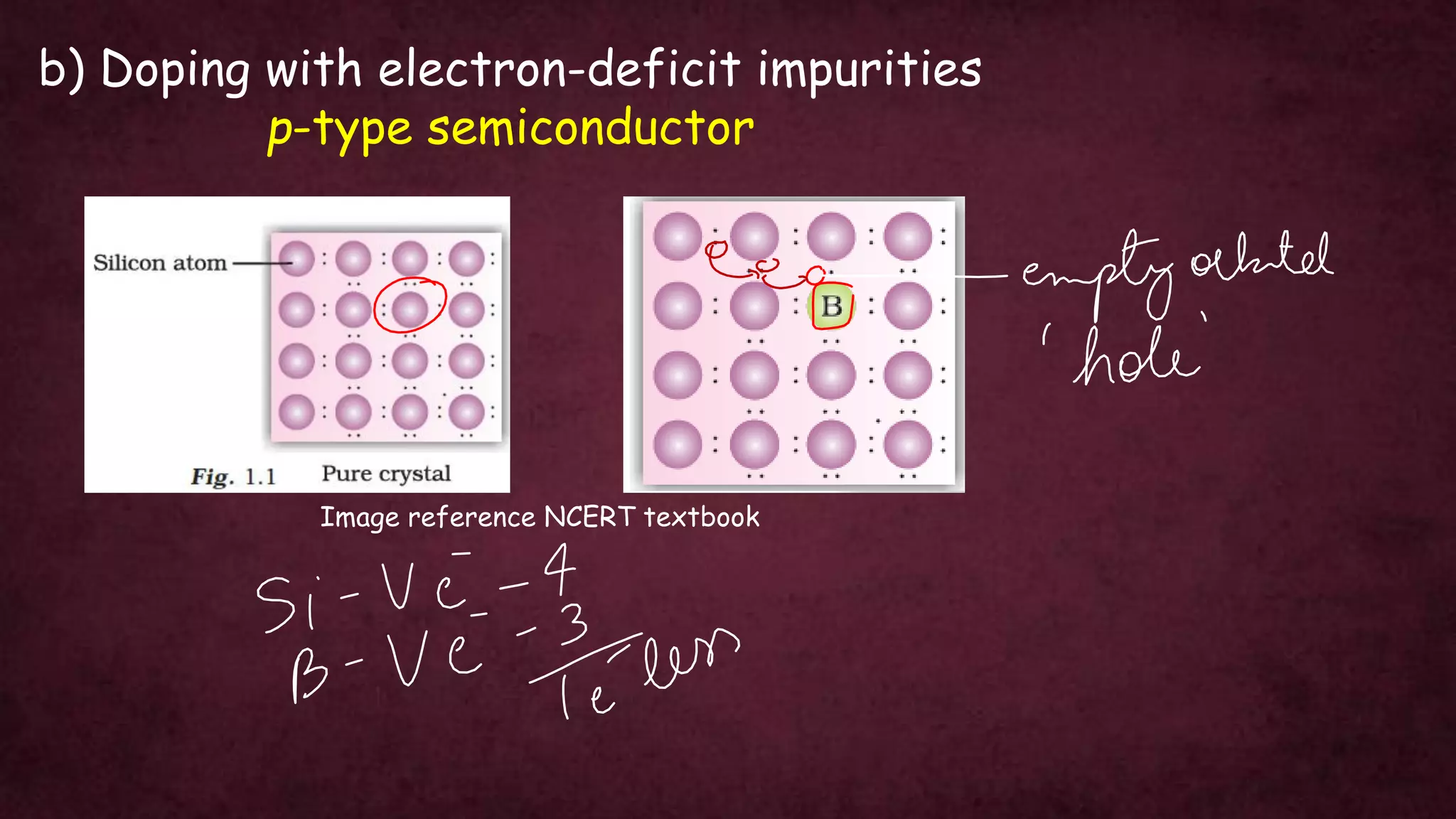
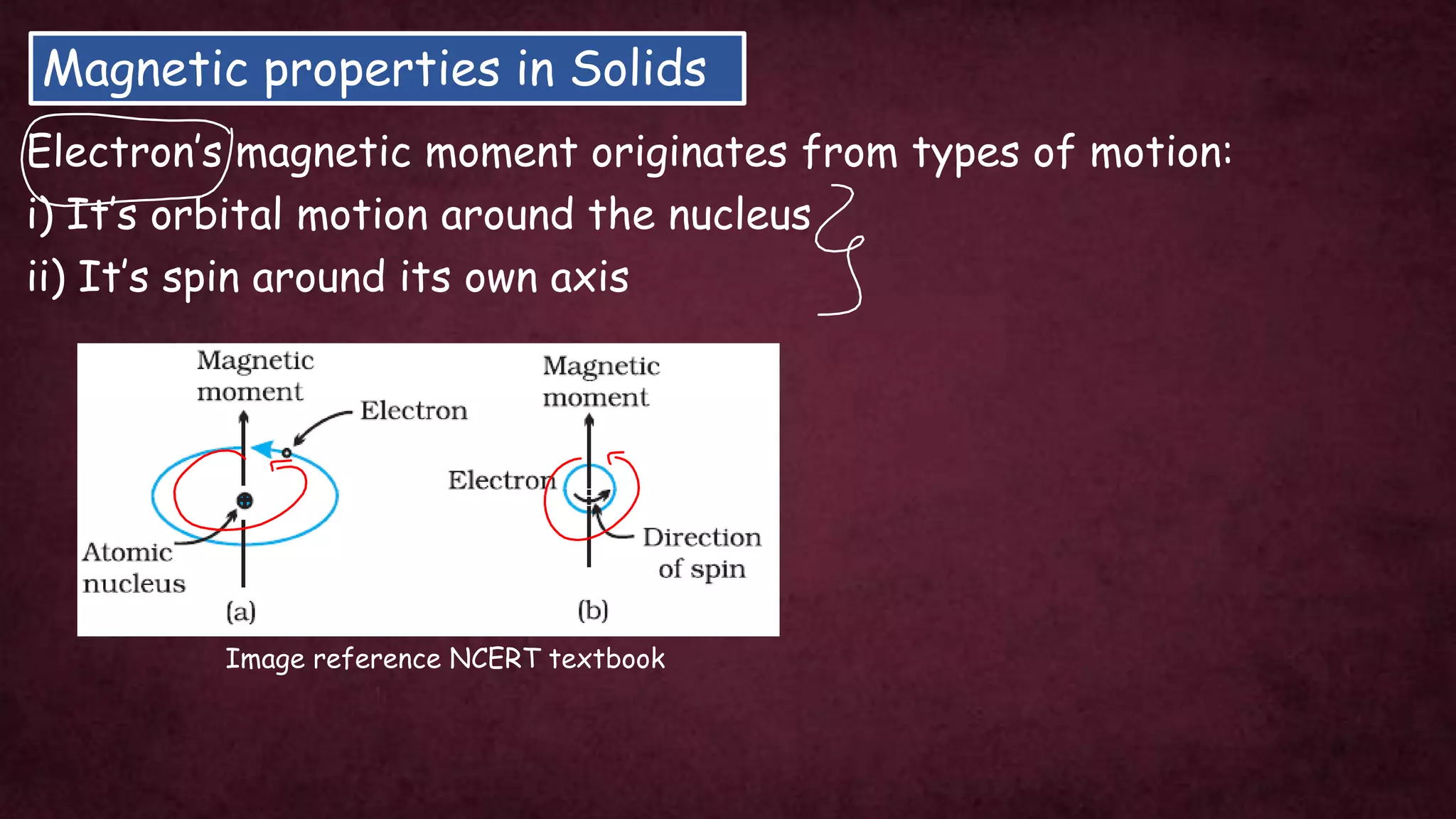
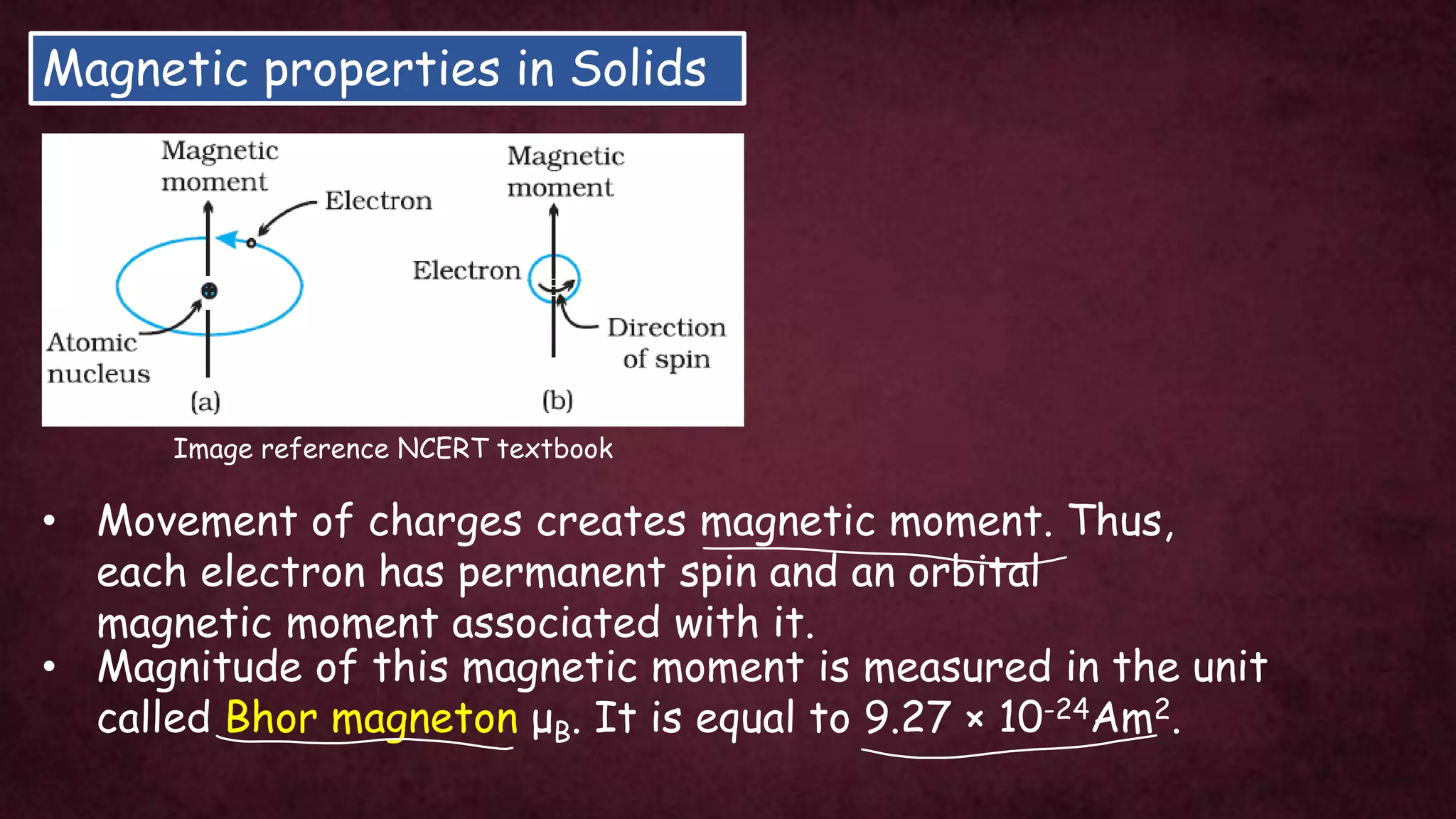
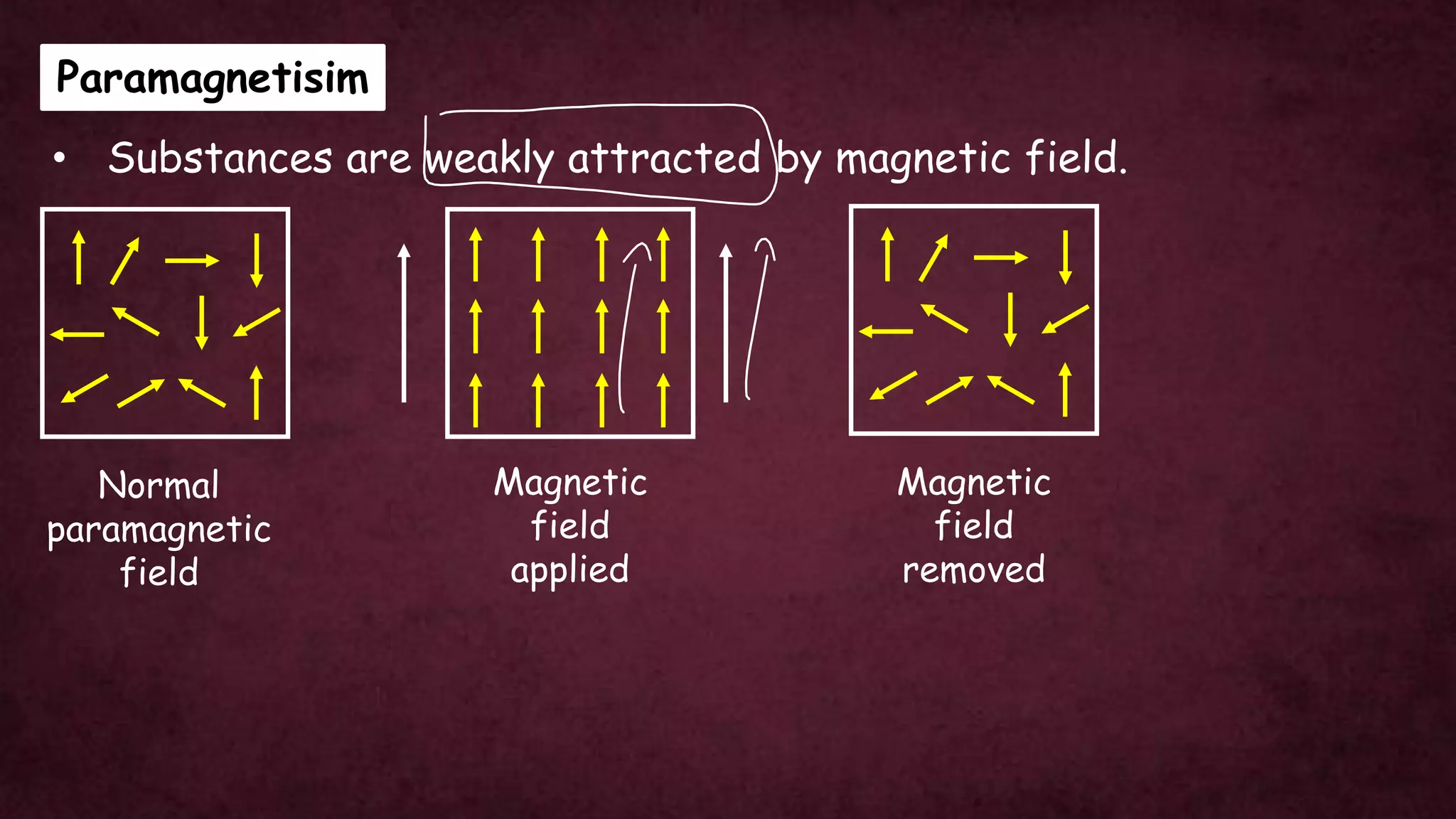
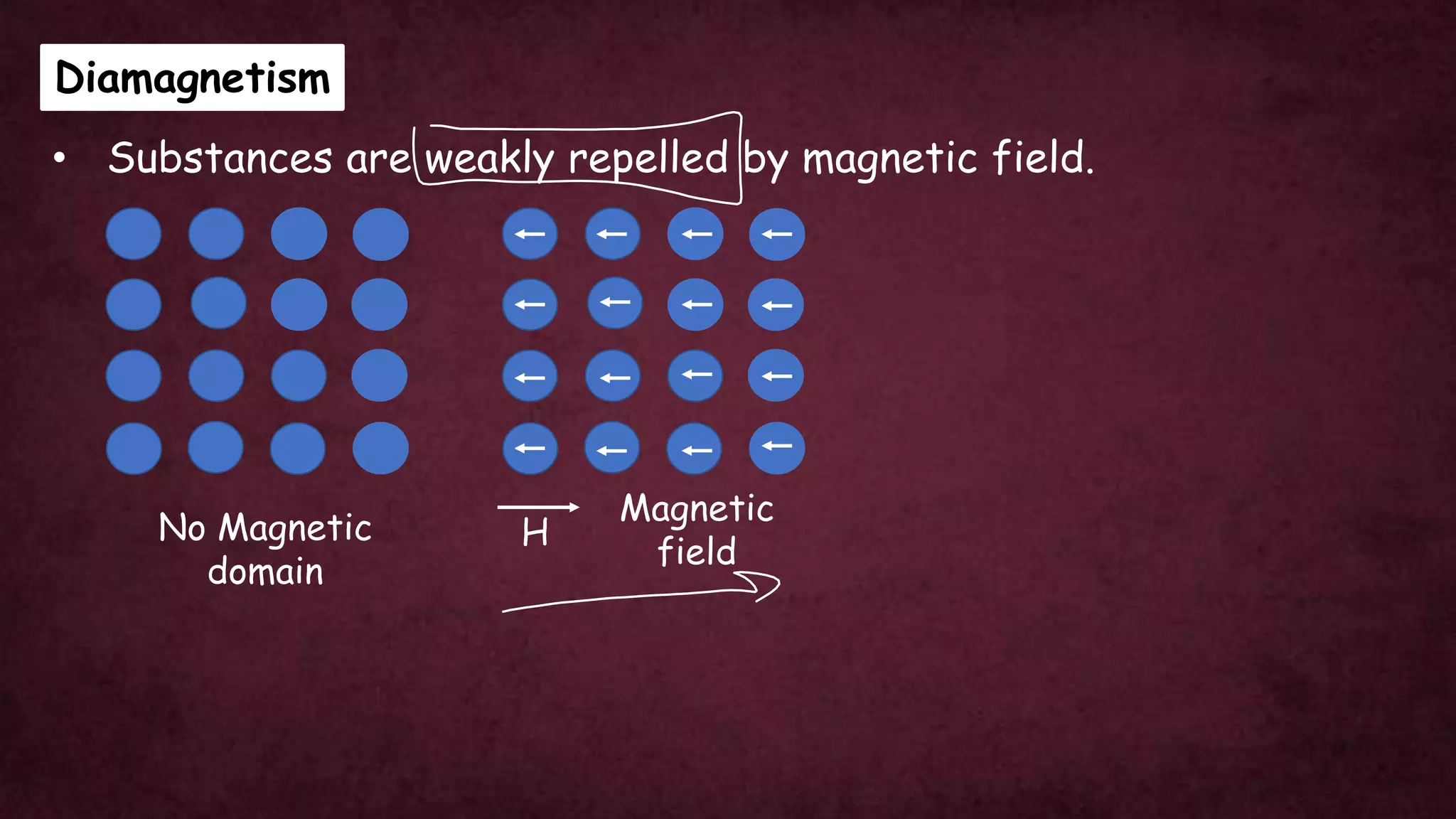
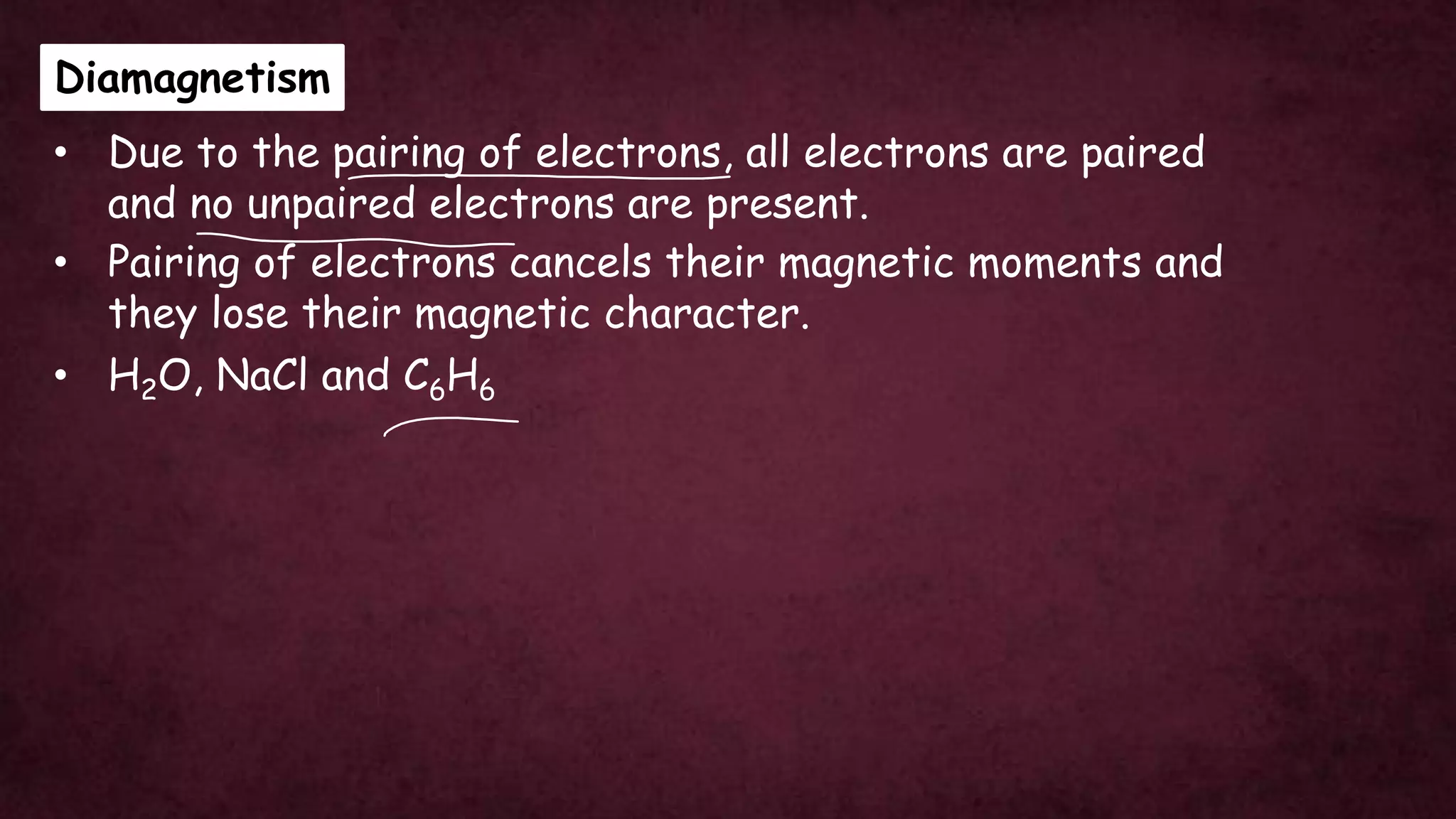
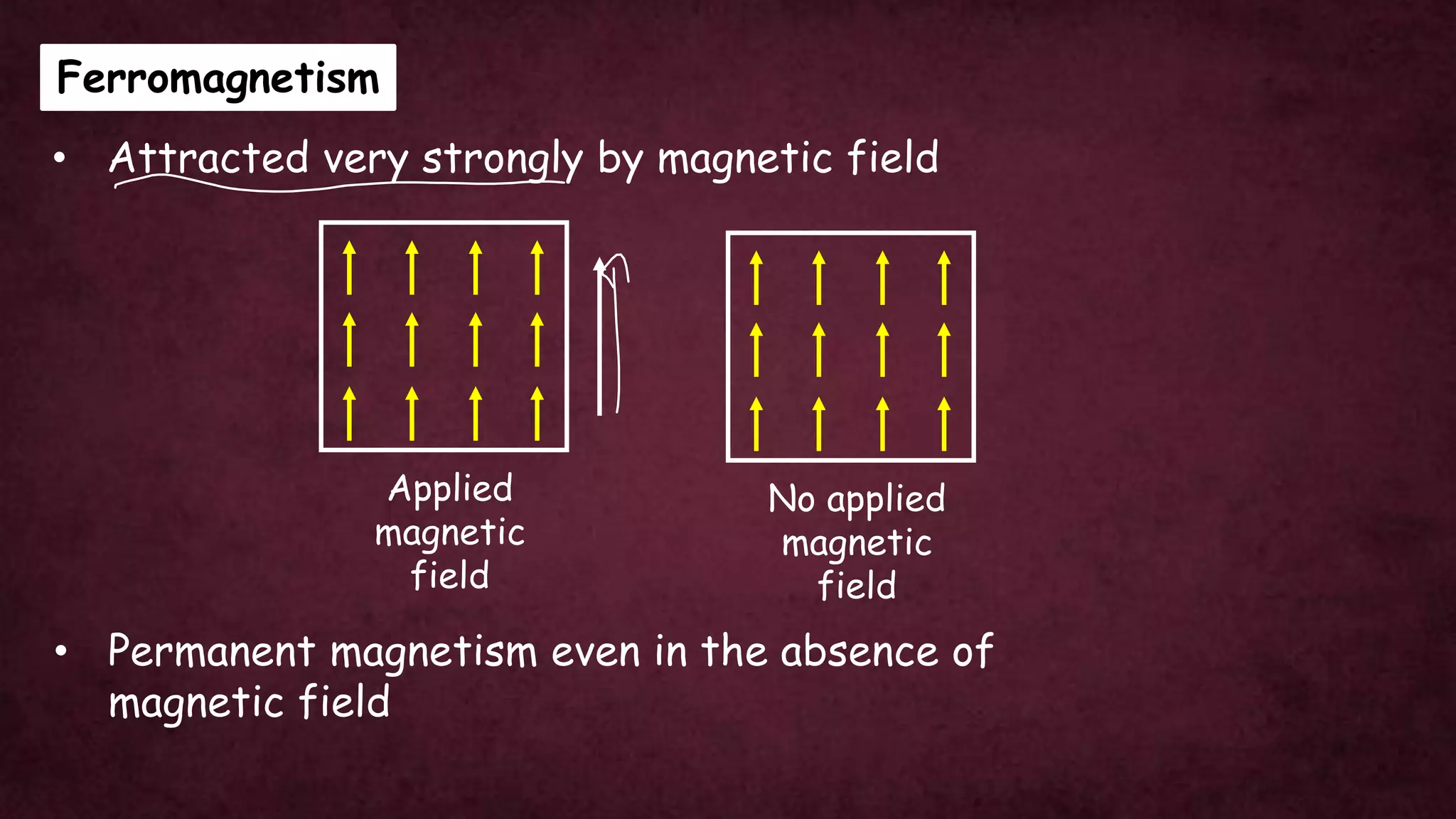
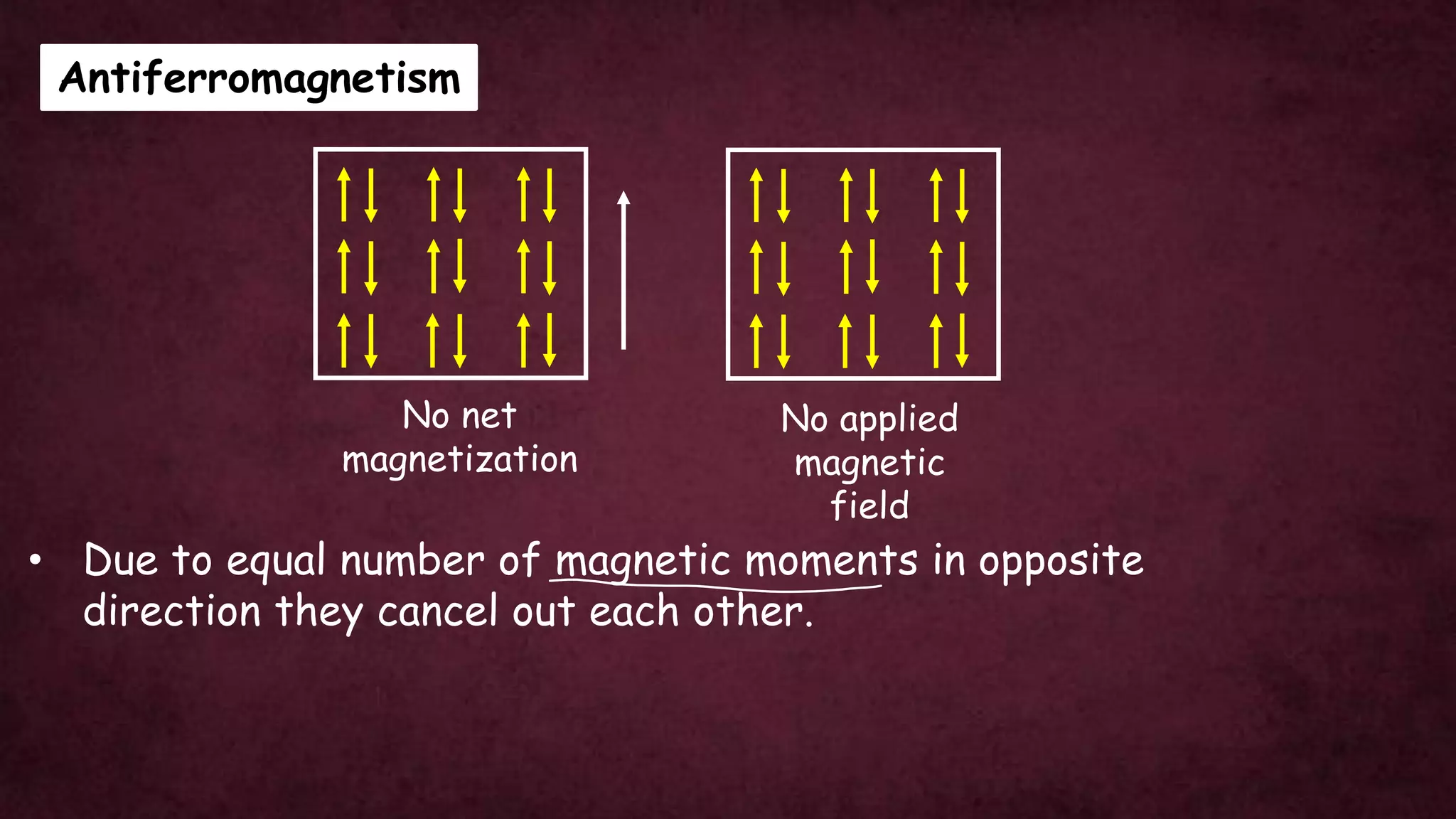
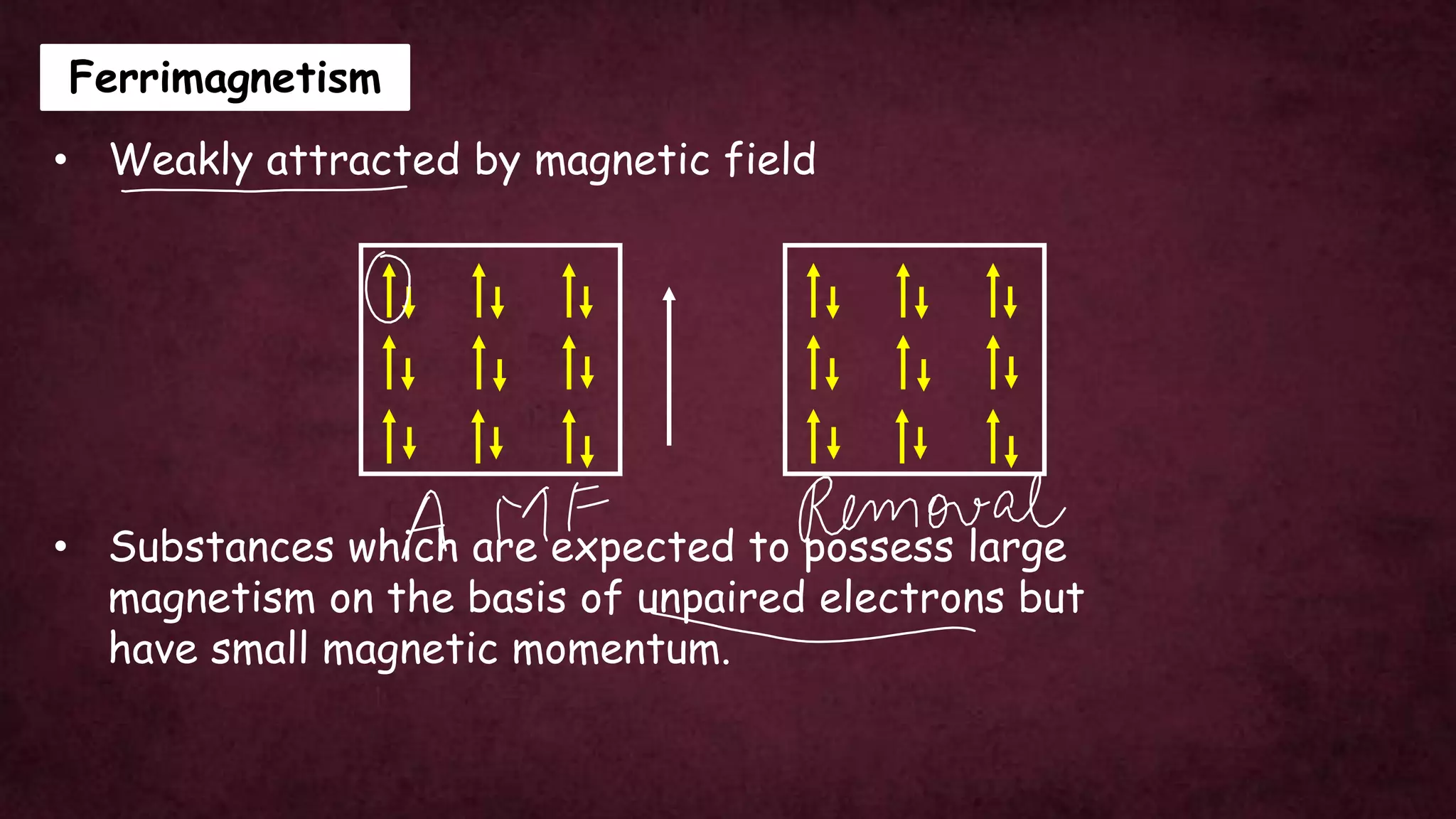
The document discusses solid-state physics, focusing on defects in solids, types of conductors (conductors, insulators, semiconductors), and the impact of doping on semiconductor conductivity. It explains magnetic properties of solids, classifying them into categories such as paramagnetic, diamagnetic, ferromagnetic, antiferromagnetic, and ferrimagnetic, with details on electron behavior and magnetic moments. Applications of n-type and p-type semiconductors, such as diodes and transistors, are also highlighted.