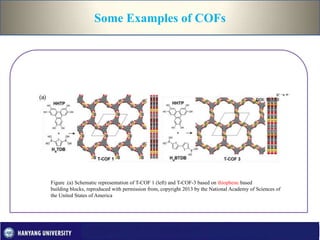
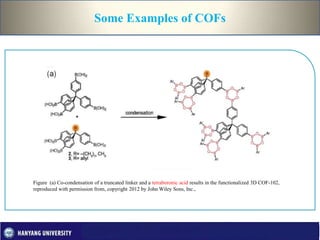
This document discusses covalent organic frameworks (COFs), which are porous organic materials constructed through strong covalent bonds between organic building units. It describes various types of bonds and introduces COFs and metal-organic frameworks. The key advantages of COFs are their covalent linkages, porosity, crystallinity, and tunable properties. Common synthesis methods for COFs include solvothermal synthesis using solvents and heat and microwave synthesis for faster reactions. Characterization techniques and examples of 2D and 3D COFs are provided. Finally, potential applications of COFs in gas storage, catalysis, semiconduction and photoconduction are outlined.