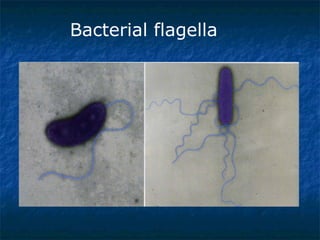
1. Curviform bacteria like Campylobacter, Helicobacter, and Vibrio can cause diseases in humans.
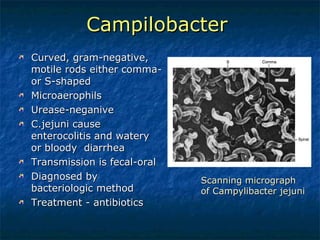
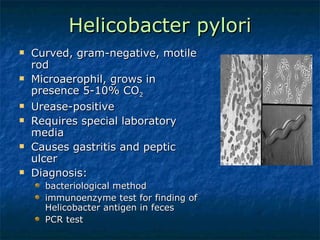
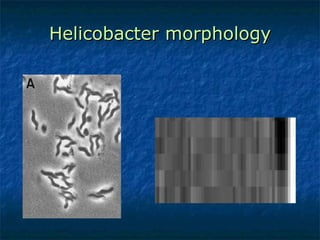
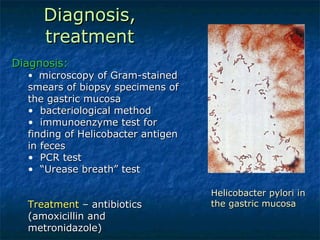
2. Campylobacter causes enterocolitis and diarrhea. Helicobacter pylori causes gastritis and peptic ulcers.
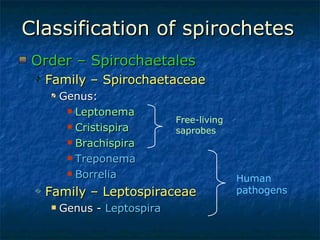
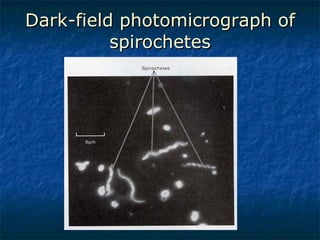
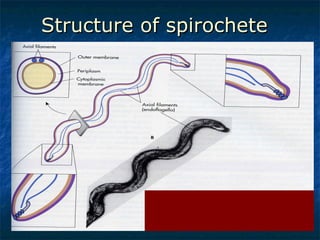
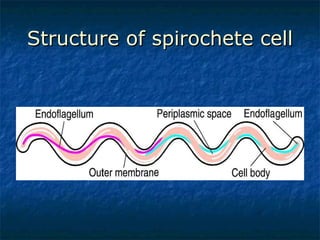
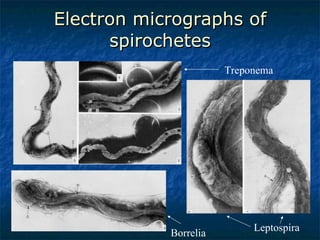
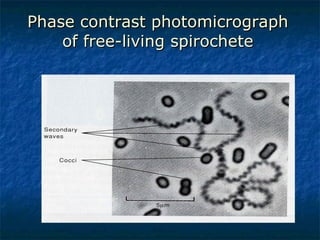
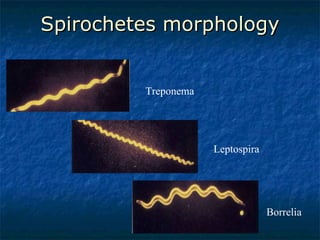
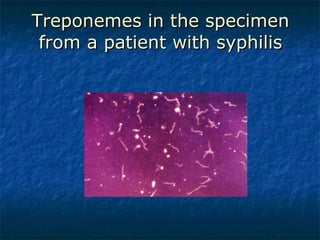
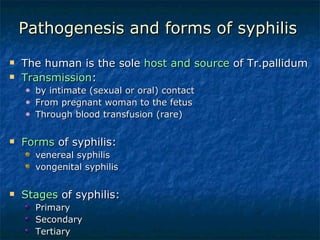
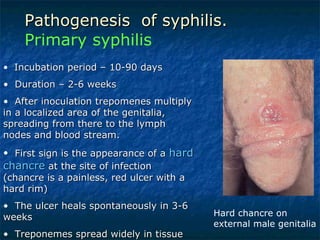
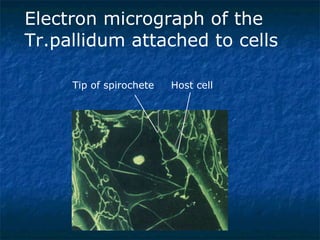
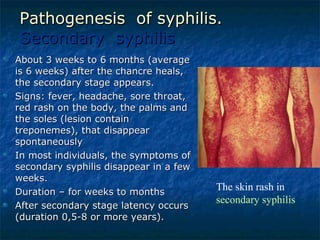
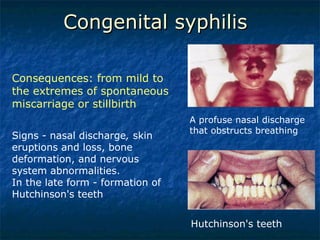
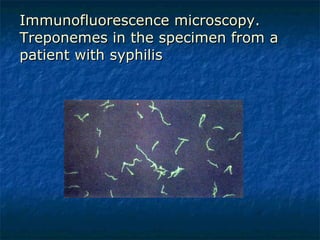
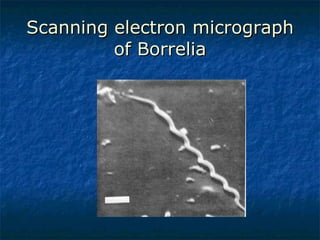
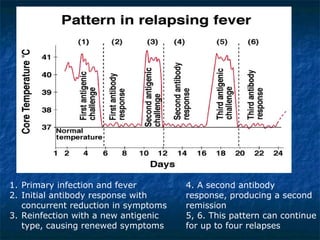
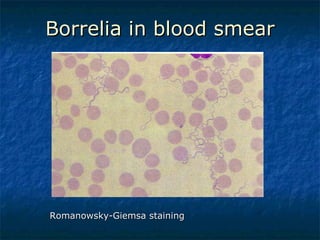
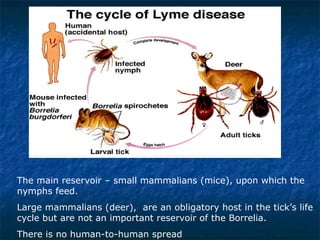
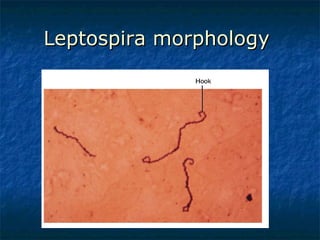
3. Spirochetes are spiral-shaped bacteria including Treponema, Borrelia, and Leptospira. Some are human pathogens that cause diseases like syphilis, Lyme disease, and relapsing fever.