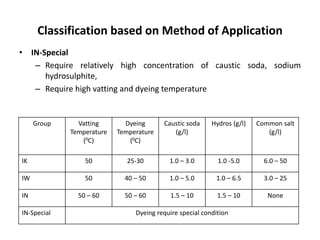
Vat dyes are insoluble in water but can be converted to soluble leuco compounds using reducing agents like sodium hydrosulphite and caustic soda. These leuco compounds are applied to cotton and oxidized back to insoluble dyes. Vat dyes are classified by application temperature and chemical requirements, with IK requiring the lowest temperatures and chemicals and IN-Special requiring the highest. The vat dyeing process involves vatting to solubilize the dye, dyeing to apply it to cotton, oxidation to fix it, and after treatment including soaping to improve fastness.




![O
O
ONa
ONa
Reduction Solubilisation
Insoluble
Vat Dye
Soluble Vat
Dye
OH
OH
Oxidation
[H]
[O]](https://image.slidesharecdn.com/12-181213103642/85/Vat-dye-6-320.jpg)




