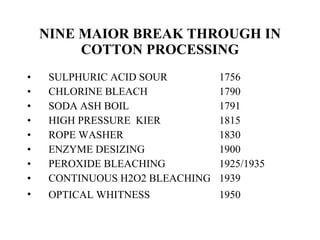
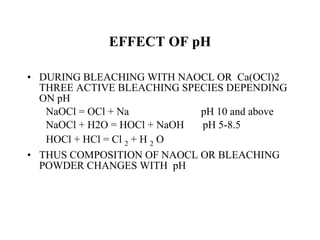
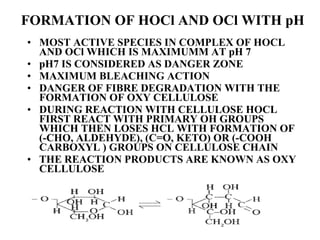
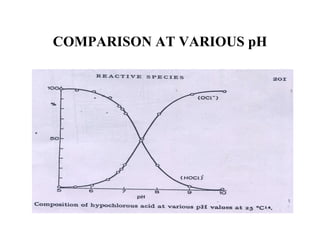
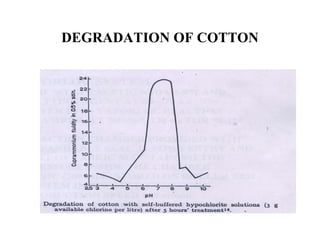
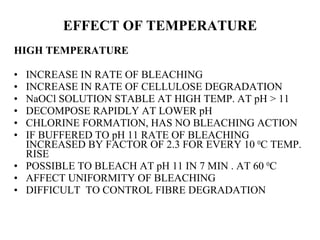
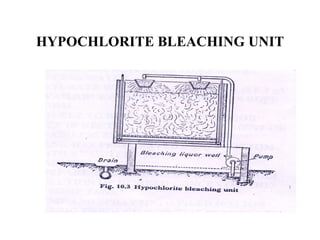
The document discusses the bleaching of cotton fabric using chlorine-based bleaching agents such as bleaching powder and sodium hypochlorite. It explains that the objective of bleaching is to produce a white fabric by destroying color compounds while minimizing fiber degradation. It then describes the manufacturing process for bleaching powder and sodium hypochlorite, and compares their properties and effects on bleaching cotton at different pH levels, temperatures, and concentrations. Finally, it outlines the typical industrial bleaching operation process.