Embed presentation




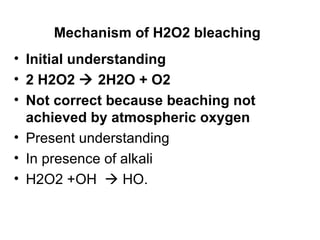

Hydrogen peroxide was discovered in 1818 and first used for cotton bleaching in 1866, though its high costs limited industrial use until 1935. It is a colorless, corrosive liquid that is stable under acid conditions but decomposes rapidly under alkaline pH, requiring stabilizers like sodium silicate to control its decomposition rate. When activated by alkalis like sodium hydroxide or carbonates, hydrogen peroxide bleaches cotton through formation of reactive radicals rather than atmospheric oxygen.




