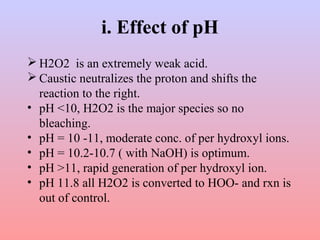
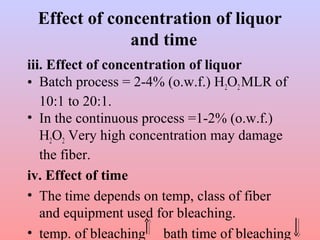
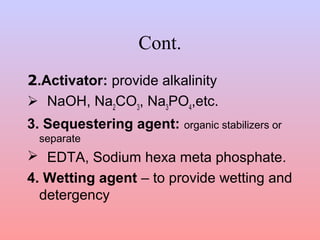
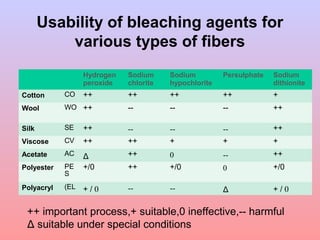
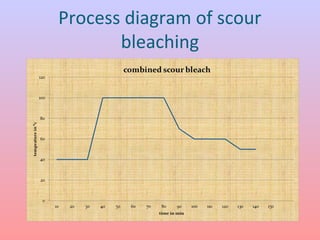
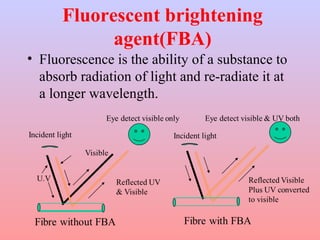
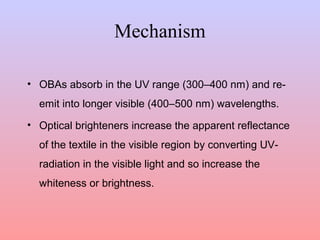
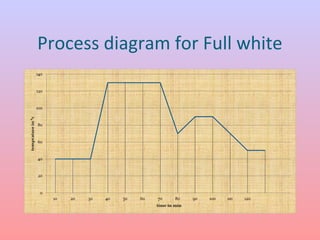
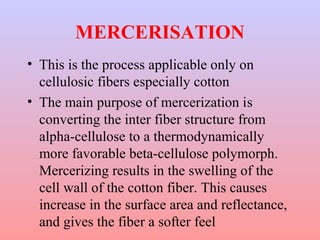
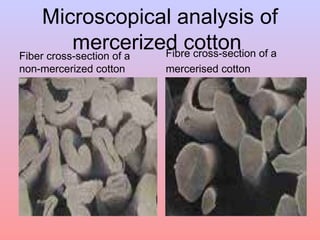
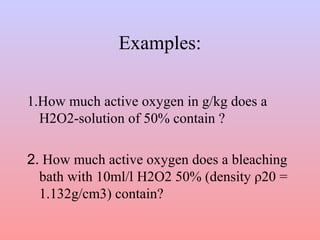The document discusses the chemical processing and pre-treatment of cotton fabrics, detailing the objectives and importance of fabric preparation to remove impurities for effective dye uptake and improved appearance. It outlines various preparatory operations such as singeing, desizing, scouring, and bleaching, including the chemicals involved and their mechanisms. The document also covers quality control measures and the effects of bleaching on fabric properties, emphasizing the balance between desired whiteness and strength retention.




































![• Out of 34.0146g H2O2 100 % result from
½ · 31.9988g =15.9994g of so-called
active oxygen.
• The conversion factor of % by weight of
H2O2-solution in active oxygen is the
following:
active oxygen [g/kg] = % in weight H2O2
solution * 4.704](https://image.slidesharecdn.com/wetprocessingofcellulosetextiles-141117024640-conversion-gate01/85/Wet-processing-of-cellulose-textiles-1-40-320.jpg)