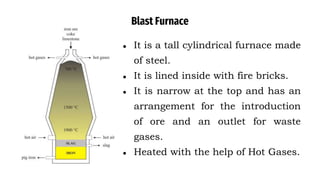
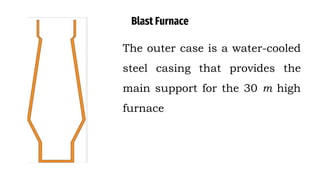
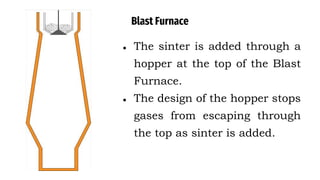
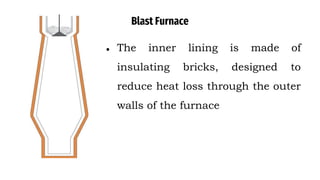
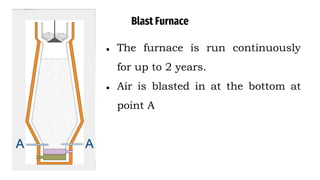
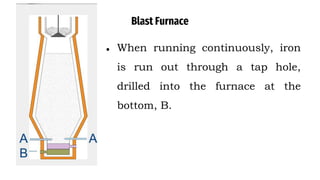
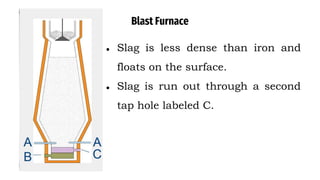
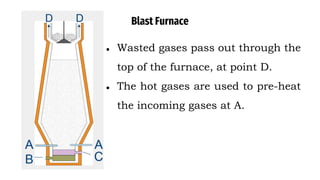
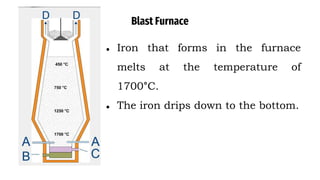
The blast furnace is a large steel tower lined with refractory brick used to reduce and convert iron oxides into liquid iron. Iron ore, coke and limestone are continuously fed into the top of the blast furnace while preheated air is blown into the bottom. Inside the blast furnace, the coke reacts with oxygen in the air to produce carbon dioxide and later carbon monoxide, which reduces the iron ore to molten iron. The molten iron and slag are drained from the bottom while waste gases exit from the top.