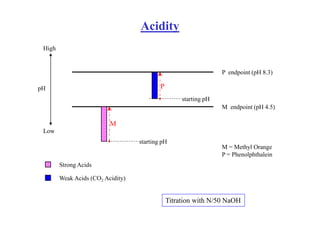
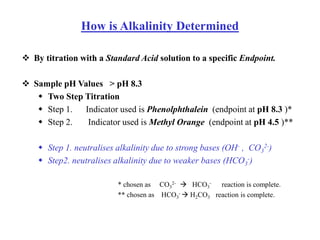
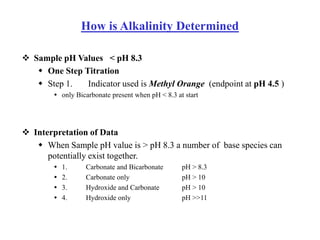
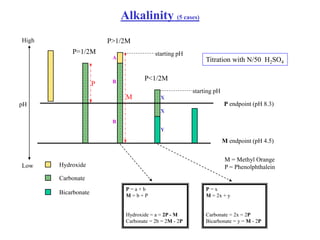
This document defines acidity and alkalinity in water samples. Acidity is the capacity to neutralize added alkalis and is determined by titration with a standard alkali solution. Alkalinity is the capacity to neutralize acids and is determined by titration with a standard acid solution. Both are usually reported as calcium carbonate equivalent. The document outlines the different types of acidity and alkalinity, how they are measured through titration, and how the results are calculated and reported. It also discusses some applications of acidity and alkalinity data.


![Equilibrium of CO2 , HCO3
- , CO3
2-
CO2 (aq) + H2O H2CO3 H+ + HCO3
- 2H+ + CO3
2-
CO2 (g)
pH 4 4.5 5 6 7 8 8.3 9 10
pKa = 6.3 pKa = 10.3
350ppm
10-5M
pH = pKa + log ([A-] / [AH])
When reaction is 99% to the left then
pH = 6.3 + log (1/100)
= 6.3 - 2
pH = 4.3
air
water](https://image.slidesharecdn.com/acidityalkalinity99-221010175722-4d43a3aa/85/acidityalkalinity-of-water-sample-4-320.jpg)