The document outlines the biopharmaceutical classification system (BCS), developed in 1995, which classifies drugs based on their aqueous solubility, permeability, and dissolution rate to aid in drug development and approval processes. It discusses factors affecting BCS, including solubility, permeability, and dissolution, as well as strategies for enhancing bioavailability and drug delivery systems, highlighting self-emulsifying and gastro-retentive drug delivery systems. The document emphasizes the significance of BCS in predicting drug performance, regulatory considerations like biowaivers, and improvements in pharmaceutical formulations.


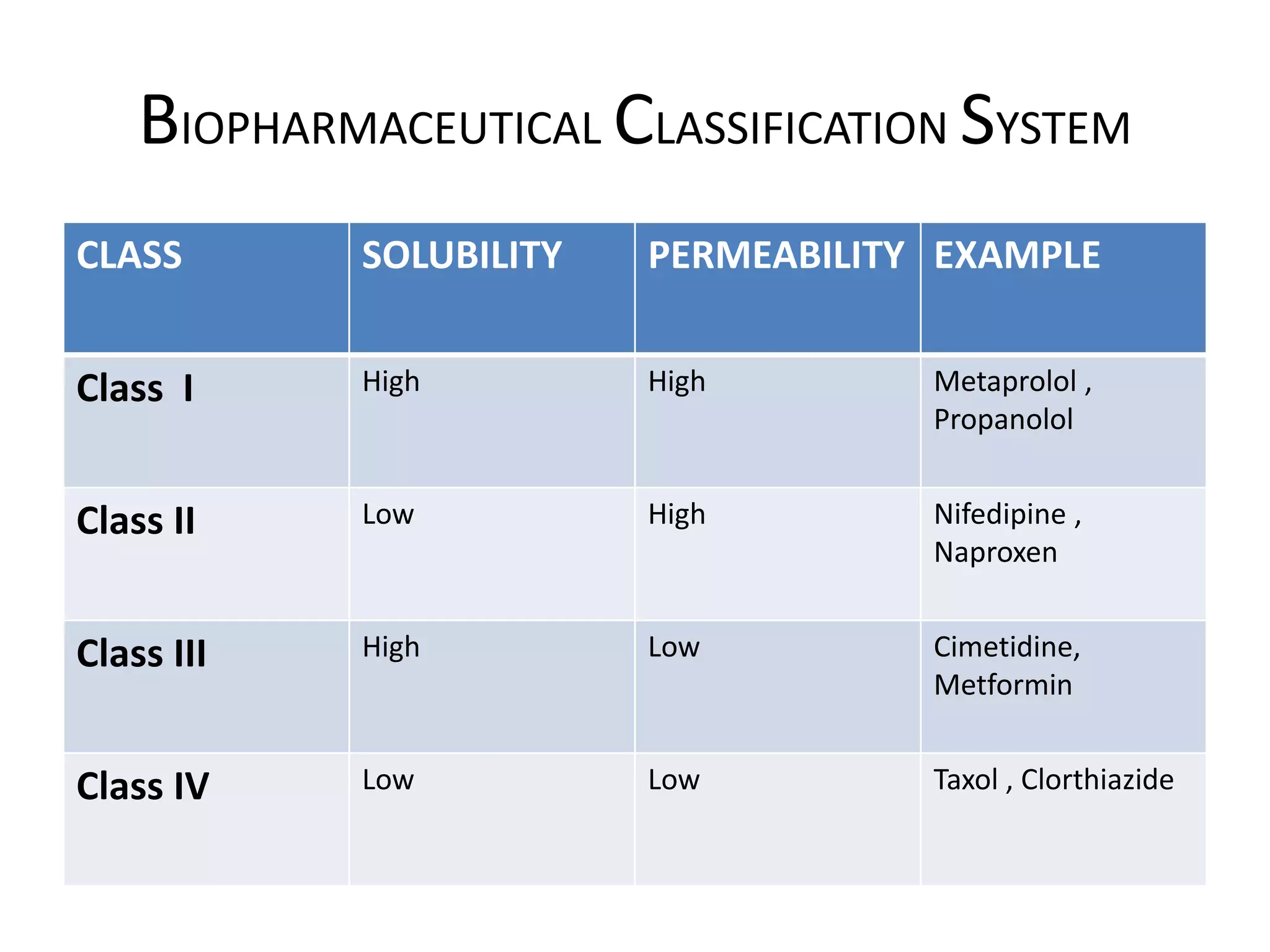
![BIOPHARMACEUTICAL CLASSIFICATION SYSTEM
• The Biopharmaceutical Classification System was
first developed by in 1995, by Amidon et al & his
colleagues.
• The Biopharmaceutical Classification System is
defined as scientific framework for classifying a
drug substance based on its aqueous solubility &
intestinal permeability & dissolution rate.
• To saved time fast screening is required so drug
substances are classified on basis of solubility and
permeability. This classification is called
Biopharmaceutical Classification System. [1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-3-2048.jpg)
![FACTOR AFFECTING BCS
• There are 3 factor affecting BCS are as follows
1. Solubility
2. Permeability
3. Dissolution
• SOLUBILITY
The Maximum Amount of solute dissolved in a given solvent
under standard conditions of temperature, pressure and pH. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-4-2048.jpg)
![• Permeability
1. Permeability of the drug to pass the biological
membrane which is the lipophilic.
2. Permeability is indirectly based on the extent of
absorption of a drug substance .
3. Drug substance is considered to be highly permeable,
when the extent of absorption in human determined
to be 90% or more of administered drug or compare
to in vivo reference dose.
• Dissolution
1. Dissolution is process in which solid substance
solubilise in given solvent i.e mass transfer from solid
surface to liquid phase. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-5-2048.jpg)
![• High Solubility
– The highest single unit dose is completely soluble in 250 ml
or less of aqueous solution at pH 1 – 7.5 (37°C)
– 250 ml: derived from typical BE study protocols that
prescribe the administration of a drug product to fasting
human volunteers with a glass (approx. 250 ml) water
• High Permeability
– According to HHS-FDA, when 90 % or more of the orally
administered dose is absorbed in the small intestine.
– Permeability can be assessed by pharmacokinetic studies
(for example, mass balance studies), or intestinal
permeability methods, e.g. intestinal perfusion in humans,
animal models, Caco 2 cell lines or other suitable,
validated cell lines. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-6-2048.jpg)
![• Dissolution:
– In three different media: pH 1.2 HCl, pH 4.5 Acetate
buffer and pH 6.8 Phosphate buffer, composition in a
paddle (50 rpm) or basket (100 rpm) apparatus at 37
°C and a volume of 900 ml
• Very Rapid Dissolution:
– An IR drug product is considered VERY RAPIDLY
DISSOLVING when >85% of the labeled amount of
drug substance dissolves within 15 minutes
• Rapid Dissolution
– An IR drug product is considered RAPIDLY DISSOLVING
when >85% of the labeled amount of drug substance
dissolves within 30 minutes. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-7-2048.jpg)
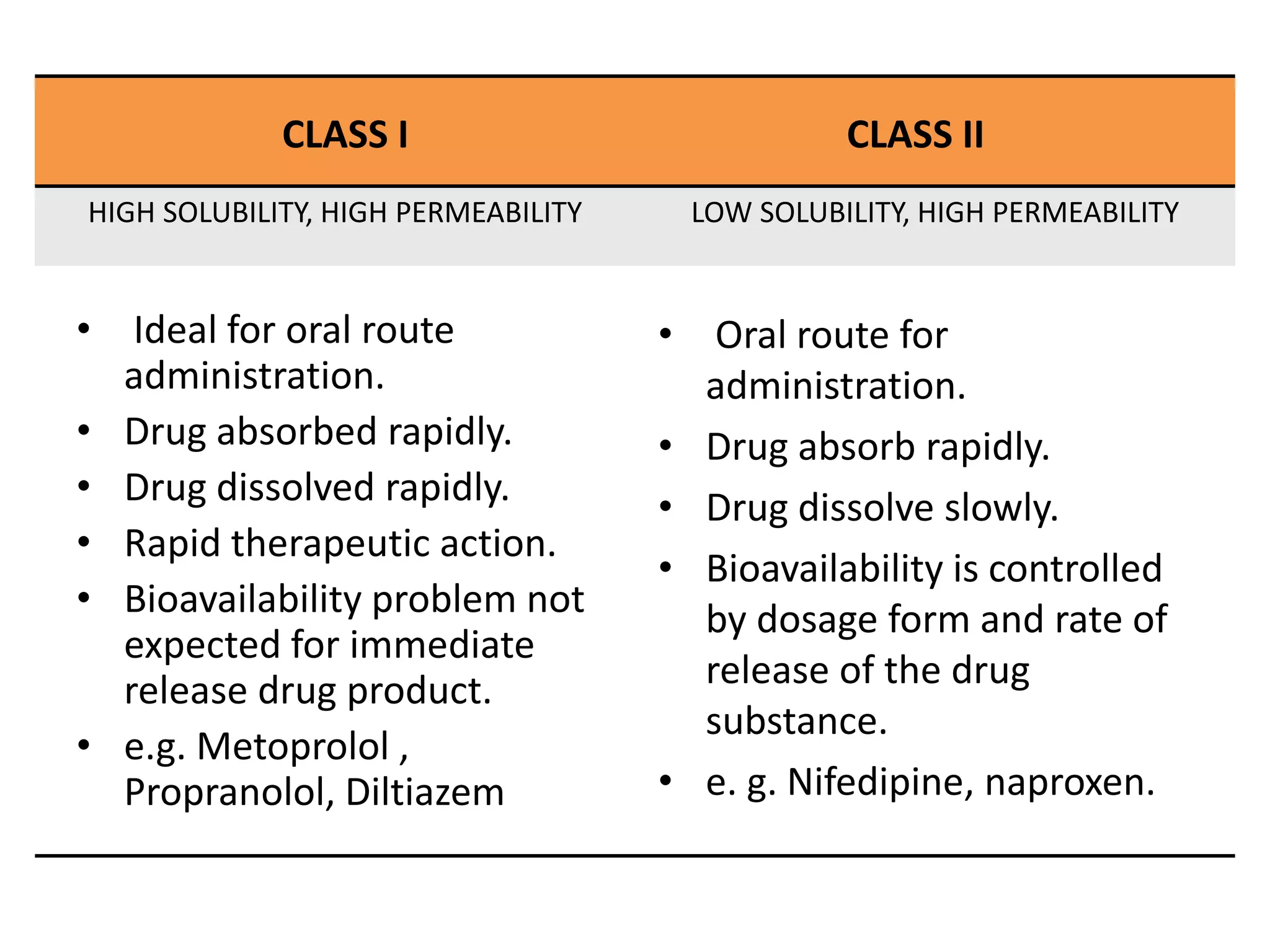
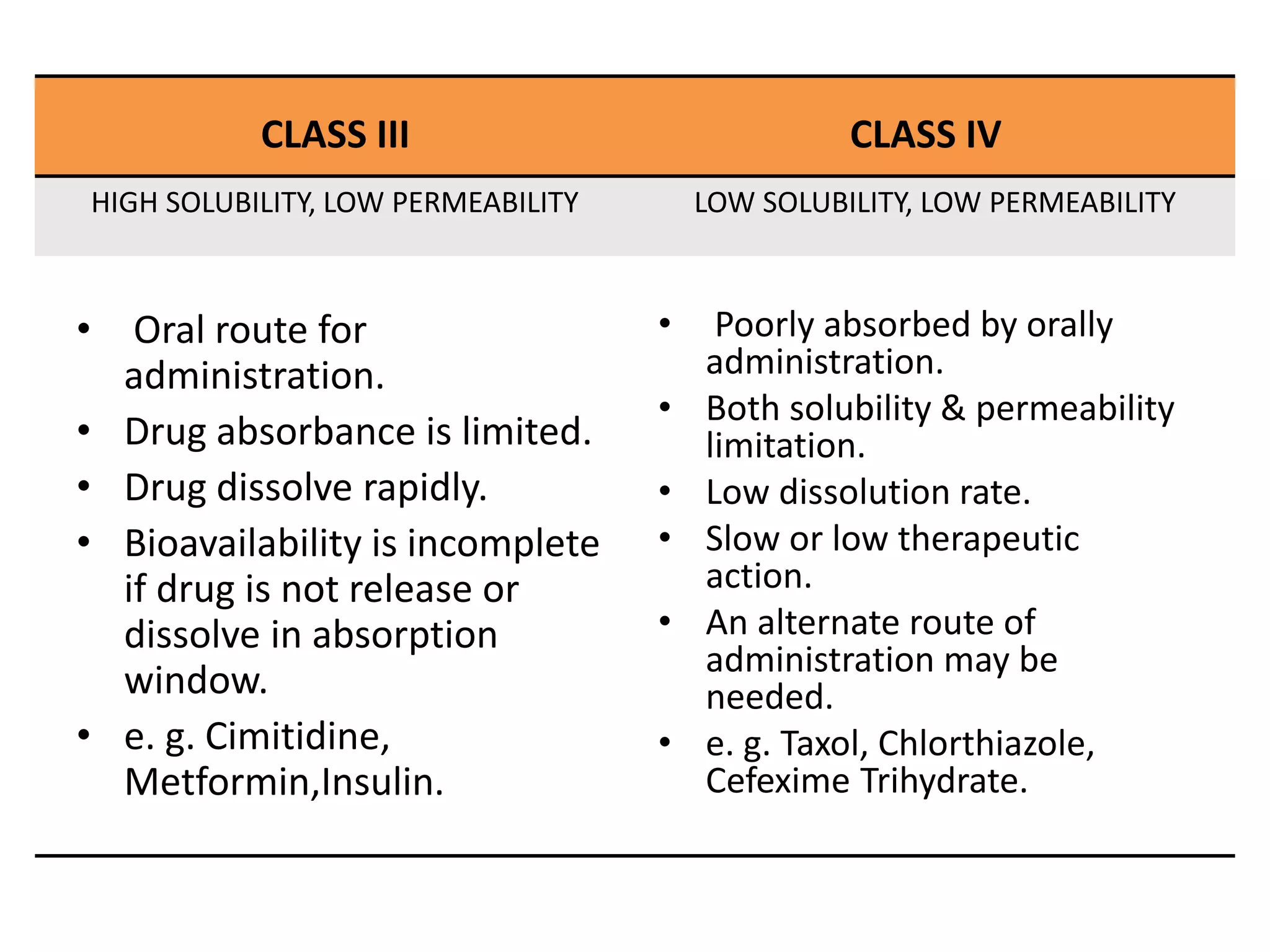
![APPLICATION
• To predict in vivo performance of drug product using
solubility and permeability measurements.
• Aid in earliest stages of drug discovery research.
• To use in biowaiver considerations.
• For research scientist to decide upon which drug
delivery technology to follow or develop.
• Also for the regulation of bioequivalence of the drug
product during scale up and post approval.[1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-11-2048.jpg)
![BIOWAIVER
• The term biowaiver is applied to a regulatory drug
approval process when the dossier (application) is
approved based on evidence of equivalence other than
through in vivo equivalence testing.
• A biowaiver means that in vivo bioavailability and/or
bioequivalence studies may be waived (i.e. not
considered necessary for product approval).
• In 1995 the American Department of Health and
Human Services, US Food and Drug Administration
(HHS-FDA) instigated the Biopharmaceutics
Classification System (BCS), with the aim of granting so-
called biowaivers for SUPACs. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-12-2048.jpg)
![BIOWAIVER
• At that time the biowaiver was only considered for
SUPAC to pharmaceutical products.
• More recently, the application of the biowaiver
concept has been extended to approval of certain
orally administered generic products
• Scope:
– Inside a product:
• Scale up processes
• Line extensions
• Variation after marketing authorisation
– between different products:
• Application of generics without clinical data. [4]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-13-2048.jpg)
![Method for enhancement of
Bioavailability
• There are 3 basic approaches are for increase
in the bioavailability are as follows
1. Pharmaceutical Approach
2. Pharmacokinetic Approach
3. Biological Approach
• Objective
1. Enhancement of drug solubility/dissolution rate
2. Enhancement of drug permeability
3. Enhancement of drug solubility
4. Enhancement of gastrointestinal retention. [1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-14-2048.jpg)
![Pharmaceutical
Approach
Pharmacokinetic
approach
Biological
approach
Modification in
the
• Formulation
• Manufacturing
process
• Physicochemical
properties
This approach
work by following
method
• Modification in
the chemical
structure
• Development of
new chemical
entity
• Prodrug Design
Biological
approach
implement the
changes in
• Route of
administration
• Solubility
• Dissolution Rate
• Permeability. [1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-15-2048.jpg)
![Methods for enhancement of bioavailability of drug by
Solubility/
Dissolution rate
Permeability
1. Micronisation
2. Nanonisation
3. Super critical fluid
recrystallization
4. Spray freeze drying
5. Evaporative precipitation
6. Use of surfactant
7. Use of salt form
8. Precipitation inhibitor
9. Alteration of pH
10.Solvent deposition
11.Selective adsorption
12.Amorphs, hydrate, solvates
• Lipid formulation
1. Lipid solutions and suspensions
2. Microemulsion
3. Solid lipid nanoparticle
4. Nanostructured lipid carriers
5. Lipid drug conjugates
6. Liposomes
• Ion Pairing
• Penetration enhancers
1. Physical
e.g. Iontophoresis, Sonophoresis,
electroporation
1. Chemical
e.g. Surfactants, azones,
Pyrrolidones, Sulphoxides.[1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-16-2048.jpg)
![Methods for enhancement of bioavailability of drug by
Drug Stability Gastrointestinal retention
• Enteric Coating
• Complexation
• Metabolism inhibitors
1. Bioadhesive delivery
system
2. Controlled release
Micro encapsulated
system
3. Immobilization of
enzyme inhibitor
• Development of Gastro
retentive drug delivery
System
• Increased Contact With
Epithelial Surfaces
• Prolonging residence time in
the Stomach
• Delaying Intestinal Transit. [1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-17-2048.jpg)
![Drug delivery system based on the BCs
• Dissolution, Permeability and solubility are 3
important factors for all kind delivery system. ( I.E. Oral,
Topical, transdermal, Sublingual, Buccal )
• Development of various Novel drug delivery system
which are as follows,
1. SEDDS (self emulsifying drug delivery system)
2. SMEDDS (self micro-emulsifying drug delivery system)
3. GRDDS (Gastro retentive drug delivery system)
4. Bioadhesive drug delivery system [1]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-18-2048.jpg)
![SEDDS / SMEDDS
Concept introduced by Hoar and Schulman in 1940’s who generated a
clear single phase solution by titrating a milky emulsion with hexanol.
Alternative names for these systems are often used, such as transparent
emulsion, swollen micelle, micellar solution, and solubilized oil.
Schulman and co-worker in 1959 subsequently coined the term Micro
emulsion.
Micro emulsions are an isotropic mixture of natural or synthetic oils, solid
or liquid surfactants, co-surfactant and drugs.
Upon mild agitation followed by dilution in aqueous media, such as
gastrointestinal (GI) fluids, the system can form fine oil in water (O/W)
micro emulsions which usually have droplet size less than 100 nm.
Micro emulsion have been successively used to improve the solubility,
chemical stability and oral bioavailability of poorly water soluble drugs.
class II & IV as per BCS classification.[3]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-19-2048.jpg)
![SEDDS
SELF EMULSIFYING DRUG DELIVERY SYSTEM
SMEDDS
SELF MICRO EMULSIFYING DRUG DELIVERY
SYSTEM
1. It is the mixture of oil,
surfactant and drug.
2. Droplet size is 100-300 nm
3. It is turbid in nature.
4. It is thermodynamically not
stable.
5. Ternary phase diagram are
used in optimization..
1. It is a mixture of oil,
surfactant, co- surfactant
and drug
2. Droplet size is less than 100
nm.
3. It is transparent in nature.
4. It is thermodynamically
stable.
5. Pseudo Ternary Phase
diagram are used for
optimization.[3]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-20-2048.jpg)
![ADVANTAGES DISADVANTAGES
1. Enhanced oral bioavailability and
AUC enabling reduction in dose
2. More consistent temporal profile
of drug absorption
3. Selective drug targeting towards
specific absorption window in GIT
4. Protection of sensitive drug from
hostile environment in gut
5. Fine oil droplets empty rapidly
from the stomach and promote
wide distribution of drug
throughout the intestinal tract at
short time period
6. Ease of manufacture and scale up
7. Potential to deliver peptides that
are processed to enzymatic
hydrolysis in GIT.
8. Useful for both solid and liquid
dosage form
1. Lack of good predicative in vitro
models for assessment of the
formulation.
2. In-vitro model needs further
development and validation
3. Different prototype lipid based
formulations need to be
developed and tested in vivo in a
suitable animal model. [3]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-21-2048.jpg)

![Gastro-retentive drug delivery system
• Oral route is extensively used, but not all drugs are
uniformly absorbed throughout GIT.
• Drugs released after absorption window has no or
negligible absorption. This can be overcome by retaining
drug in stomach.
• Gatro rententive drug delivery (GRDDS) is one of the site
specific drug delivery for the delivery of drugs at stomach.
• It is obtained by retaining dosage form into stomach and
drug is being released at controlled manner at specific site.
• GRDDS is an approach to prolong gastric residence time,
there by targetting site specific drug release in the upper
gastrointestinal tract (GIT) for local and systemic effect.
3COPS DSU Department of Pharmaceutics.
• In GRDDS, Class 2 drugs.[2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-23-2048.jpg)

![Drug candidates not suitable for
GRDDS
• Drugs that have very limited acid solubility.
– E.g. Phenytoin
• Drugs that suffer instability in the gastric
environment.
– E.g. Erythromycin
• Drugs intended for selective release in the colon.
– E.g. 5-Amino salicylic acid and corticosteroids, etc.
• Drugs having extensive first pass metabolism. [2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-25-2048.jpg)
![FACTORS AFFECTING THE GRDDS
1. Density
2. Size and Shape of the dosage form
3. Single or Multi unit formulation
4. Age
5. Gender
6. Body posture
7. Frequency of intake
8. Diseased state of an individual. [2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-26-2048.jpg)
![ADVANTAGES DISADVANTAGES
1. Improved drug absorption because of
increased GRT.
2. Enhanced bioavailability.
3. Controlled drug delivery.
4. Reduced dosing frequency.
5. Ease of administration.
6. Better patient compliance.
7. Targeted therapy for local ailments in
the upper GIT.
8. Reduced fluctuations of drug
concentration.
9. Delivery of drugs with narrow
absorption window in small intestine
region.
1. Retention in stomach is not desirable
for drugs that cause gastric
lesions/irritations. e.g. NSAIDS.
2. Drugs degraded in the acidic
environment of stomach. e.g. insulin.
3. Drugs undergo significant first- pass
metabolism. e.g. nifedipine.
4. Drugs have limited acid solubility.
e.g.phenytoin.
5. These systems require a high level of
fluid in the stomach for drug delivery
to float and work efficiently.
6. These systems do not offer significant
over the conventional dosage forms
for drugs, which are absorbed
throughout GIT. [2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-27-2048.jpg)



![IN VITRO TEST IN VIVO TEST
1. Floating lag time
2. Floating time
3. Dissolution study
4. Swelling index
5. Muco adhesive test
6. Density
1. Radiology
2. Scintigraphy
3. Gastroscopy
4. Magnetic marker
monitoring
5. Ultrasonography. [2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-31-2048.jpg)

![APPLICATION
Enhanced bioavailability
Sustained drug delivery
Site specific drug delivery system
Absorption enhancement
Minimized adverse activity at the colon
Reduced fluctuation of drug concentration. [2]](https://image.slidesharecdn.com/biopharmaceuticalclassificationsystem-210620052228/75/Biopharmaceutical-classification-system-drug-delivery-system-associated-with-it-33-2048.jpg)

