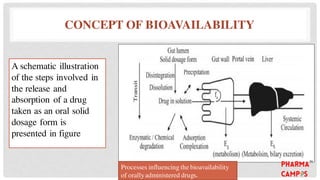
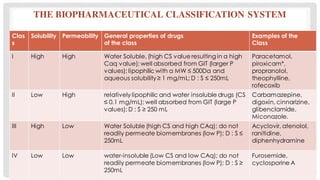
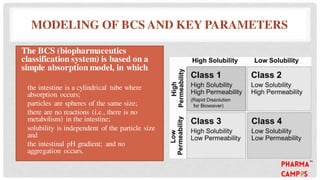
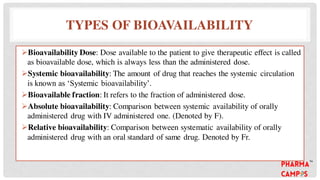
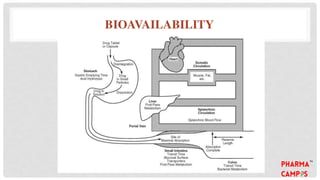
Bioavailability refers to the fraction of an extravascular dose reaching systemic circulation, impacted by factors such as drug solubility and permeability. The Biopharmaceutical Classification System (BCS) categorizes drugs into four classes based on their solubility and absorption characteristics, aiding in the development of strategies to improve bioavailability. Bioavailability studies aim to evaluate new drug entities, the influence of excipients, and the effects of various patient-related and processing factors.