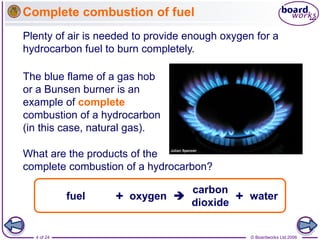
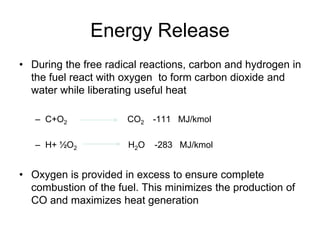
Combustion is the rapid reaction between a substance and oxygen that releases heat and light energy. Complete combustion of hydrocarbons produces carbon dioxide and water, while incomplete combustion occurs when there is insufficient oxygen and produces carbon monoxide and soot as well. Combustion technologies for biomass depend on factors like the fuel type, size, moisture content, and intended application. Solid biomass combustion in domestic applications uses stoves, while larger systems above 1 MWth are used for industrial plants.