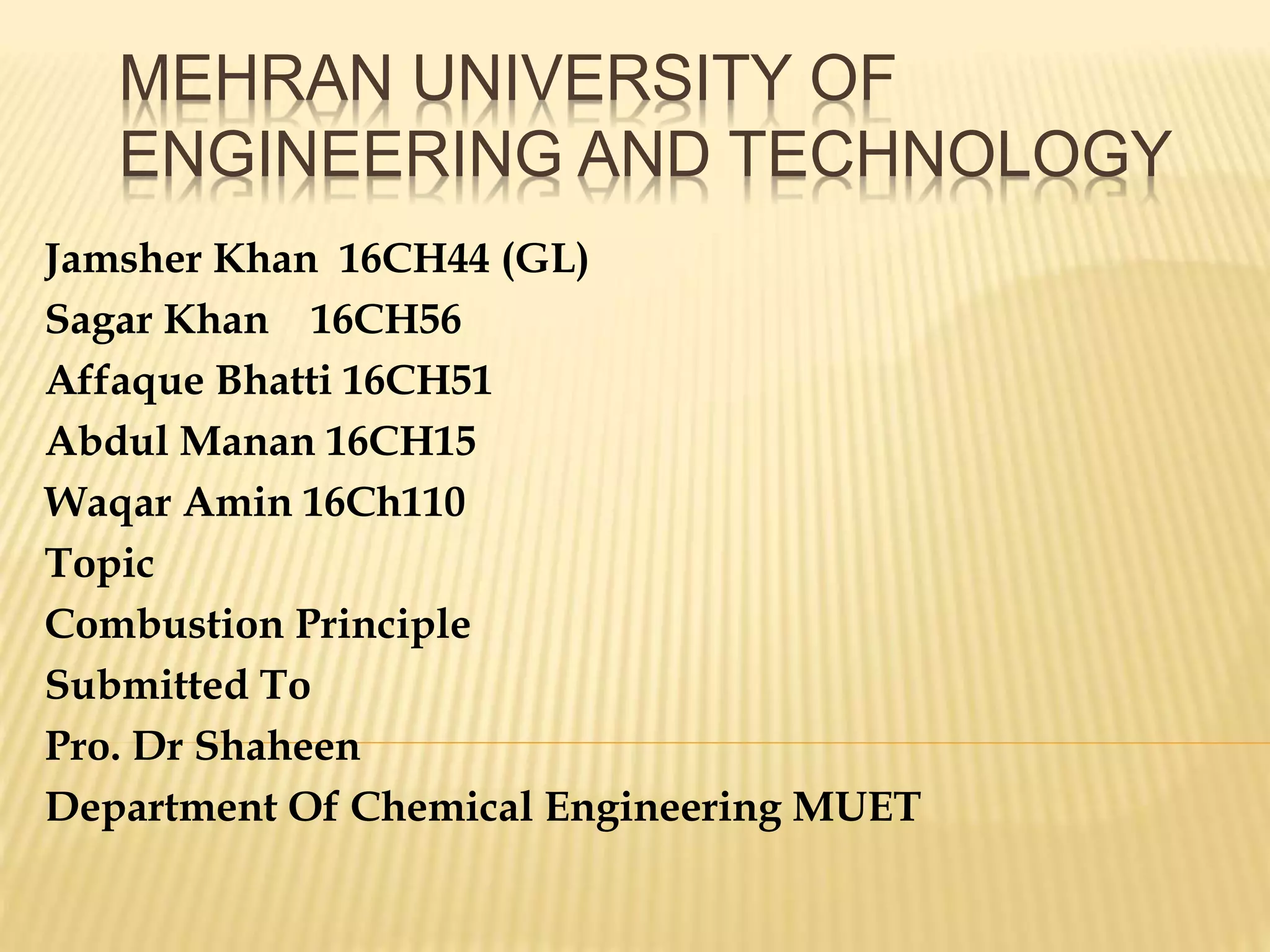
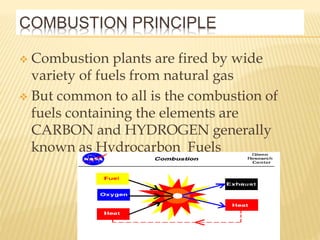
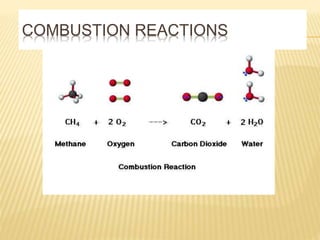
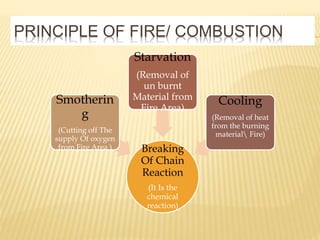
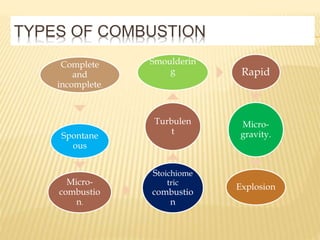
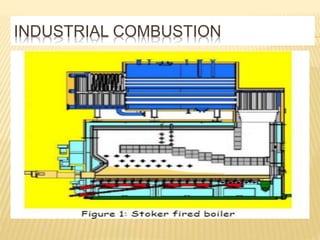
This document discusses combustion principles and provides details on various topics related to combustion. It defines combustion as a chemical reaction between a fuel and oxygen that produces a considerable amount of heat. It also discusses the basic elements required for combustion, different phases and types of combustion, industrial combustion processes and fuels, classification of industrial combustion, and concludes with emphasizing the importance of flames and combustion beds in industrial processes.