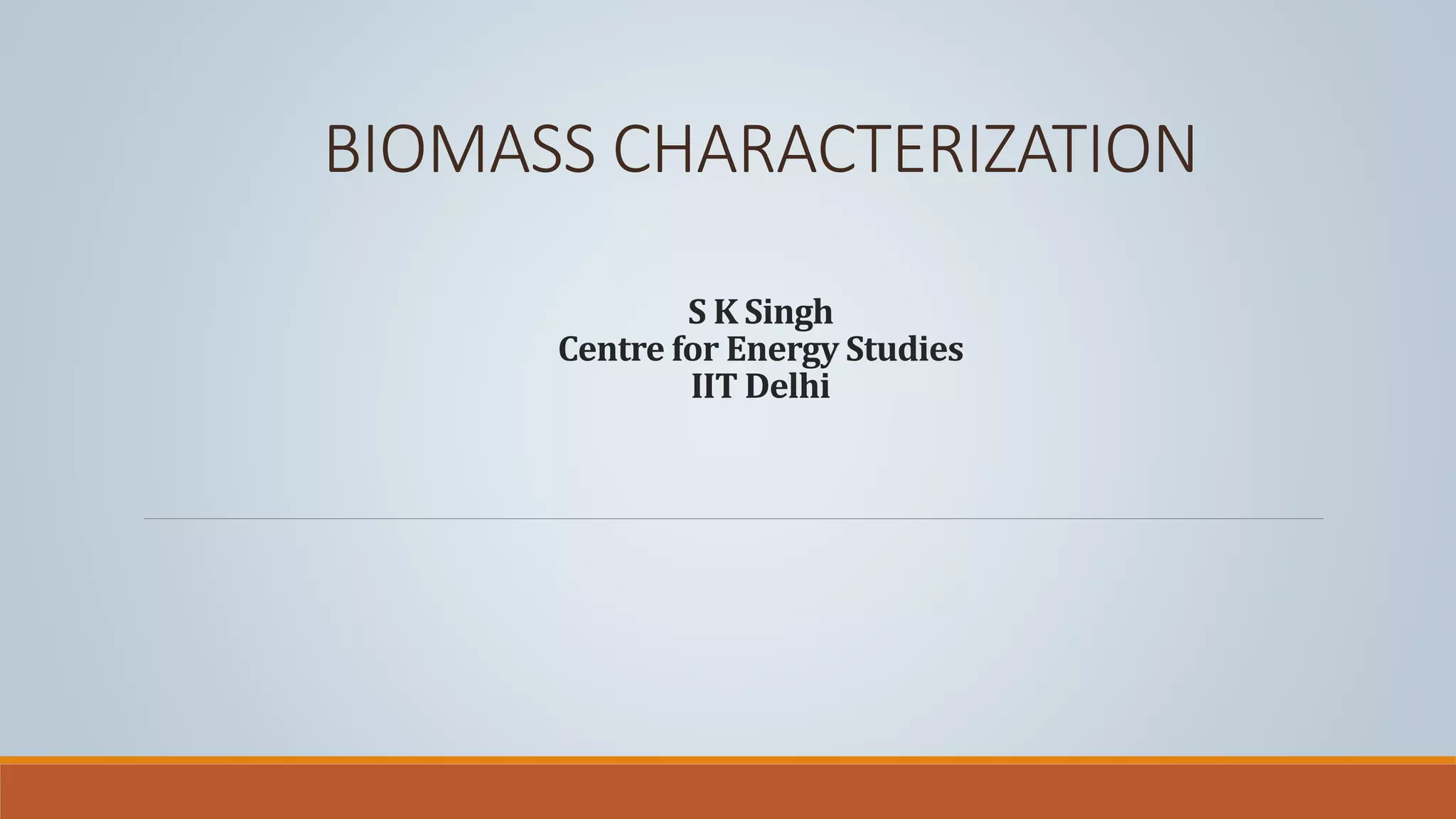
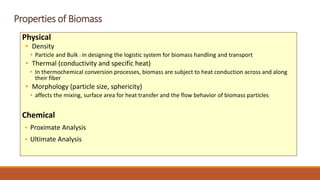
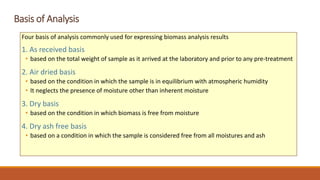
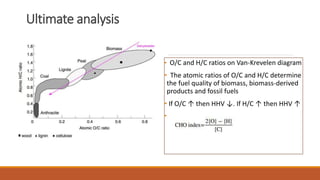
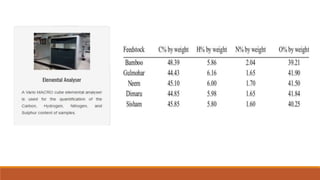
This document discusses methods for characterizing biomass, including proximate analysis, ultimate analysis, and heating value determination. Proximate analysis measures the biomass moisture content, volatile matter, ash content, and fixed carbon. Ultimate analysis determines the biomass composition of carbon, hydrogen, nitrogen, sulfur, and oxygen. Heating value represents the heat released during combustion and is usually expressed as higher or lower heating value. Characterizing biomass through these analytical methods provides essential data for designing biomass conversion processes.