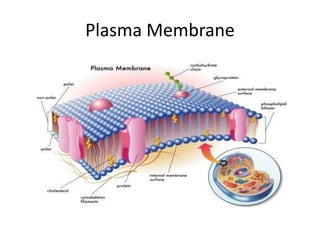
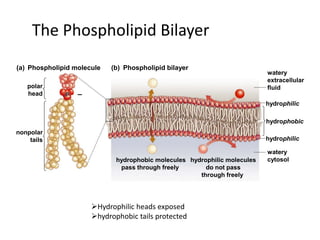
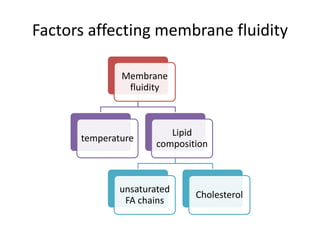
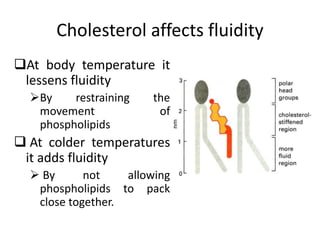
The document discusses the structure and composition of plasma membranes, describing the phospholipid bilayer and various integral and peripheral proteins that make up the fluid mosaic model. It explains the functions of cell membranes, including transport, signaling, and cell-cell interactions, and how different proteins are involved in processes like transport, reception, enzymatic activity, and adhesion. The fluidity and dynamics of the membrane are influenced by the phospholipids and cholesterol that make up the bilayer.