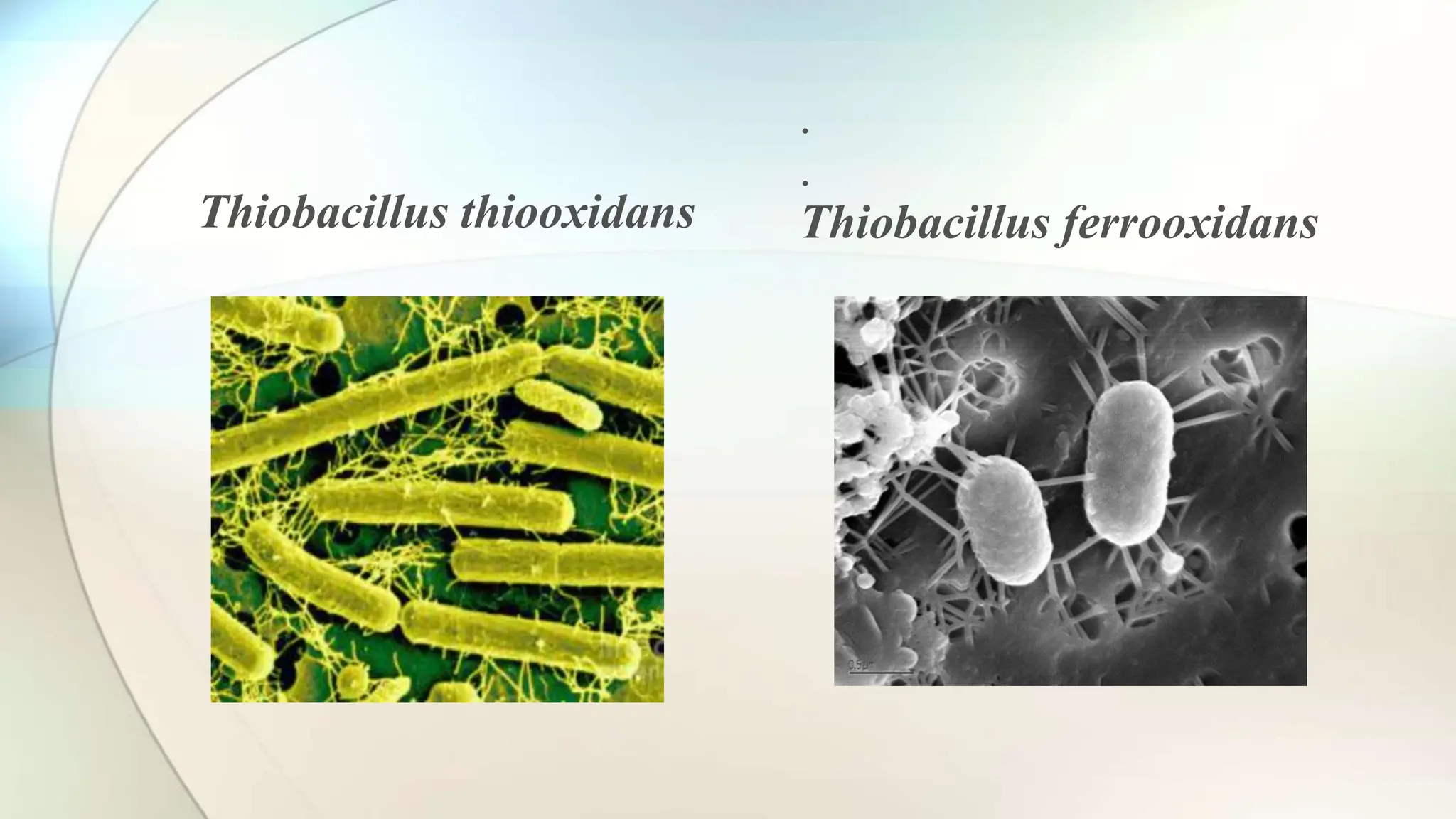
Bioleaching is a microbial process that extracts metals from ore using bacteria, and has been in use for thousands of years for metals like copper, gold, and uranium. The process is optimized commercially through controlled environmental conditions and can utilize techniques such as heap leaching and in-situ leaching. Advantages of bioleaching include cost-effectiveness and environmental friendliness, while its drawbacks are lengthy processing times and inconsistent yields.