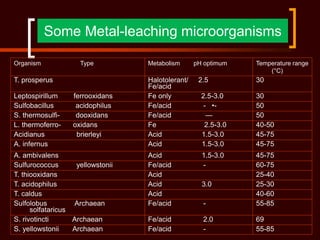
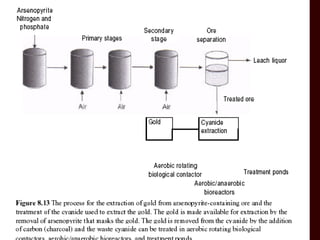
Biomining and bioleaching use microorganisms like Thiobacillus ferrooxidans to extract metals from ores and mine tailings. These microbes facilitate metal extraction by oxidizing metals or the minerals containing them, making the metals soluble so they can be recovered. Key applications include extracting copper, gold, and uranium, as well as remediating acid mine drainage. As high-grade surface deposits diminish, biomining will become increasingly important for recovering metals from lower-grade ores in a more sustainable and cost-effective manner than conventional mining and extraction methods.