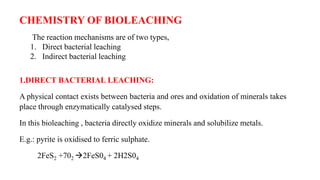
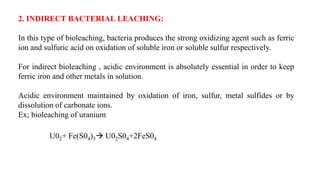
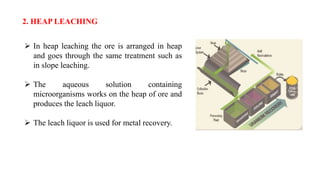
The document is a seminar paper on bioleaching written by Pavan R. that details the process of extracting metals from low-grade ores using microorganisms. It covers the types of bioleaching, microorganisms used, the chemistry involved, specific examples of metal extraction (such as copper, gold, and uranium), and discusses the advantages and disadvantages of the method. Bioleaching is presented as an environmentally friendly and cost-effective alternative to traditional mining processes.







![ Gold ores: calaverite (AuTez), sylvanite (Ag. Au) Te2, petzite
(AggAuTe2)
From low-grade sulfidic ores, gold cannot be extracted.
Gold ores need to be pretreated by roasting or by pressure
oxidation to free the gold prior to cyanide leaching.
These pretreatment is costly. After this, 70-95% of the gold in
the ore can be recovered by cyanide leaching process.
Sodium cyanide leaching process converts gold to a soluble
cyanide complex.
4Au + 8NaCN + 2H20 + 02 --> 4Na[Au(CN)2] +4NaoH
2Na[Au(CN)2 +zn -> Na2 [Zn(CN)4] + 2AU
Bioleaching occurs in reactors or heap leaching process using
Thiobacillus ferrooxidans.
After leaching ,it converts the porous ore of exposed gold for
cyanide leaching.
BIOLEACHING OF GOLD](https://image.slidesharecdn.com/bioleachingfinal-220830180701-8be27d18/85/bioleaching-11-320.jpg)







