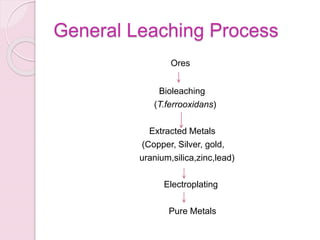
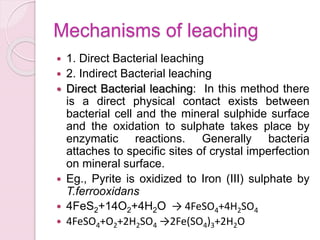
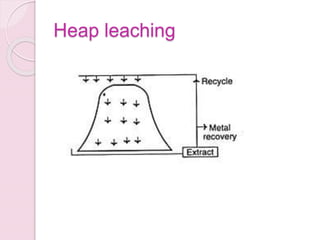
Bioleaching, also known as biomining or microbial leaching, is the process of extracting metals like copper, gold, and uranium from ores using microorganisms. Important microorganisms used in bioleaching include Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Bioleaching involves either direct contact between bacteria and mineral surfaces, or indirect leaching where bacteria generate chemical oxidants. Major industrial processes for bioleaching include heap, dump, and in situ leaching. Bioleaching provides an environmentally friendly alternative for extracting metals from low-grade ores.