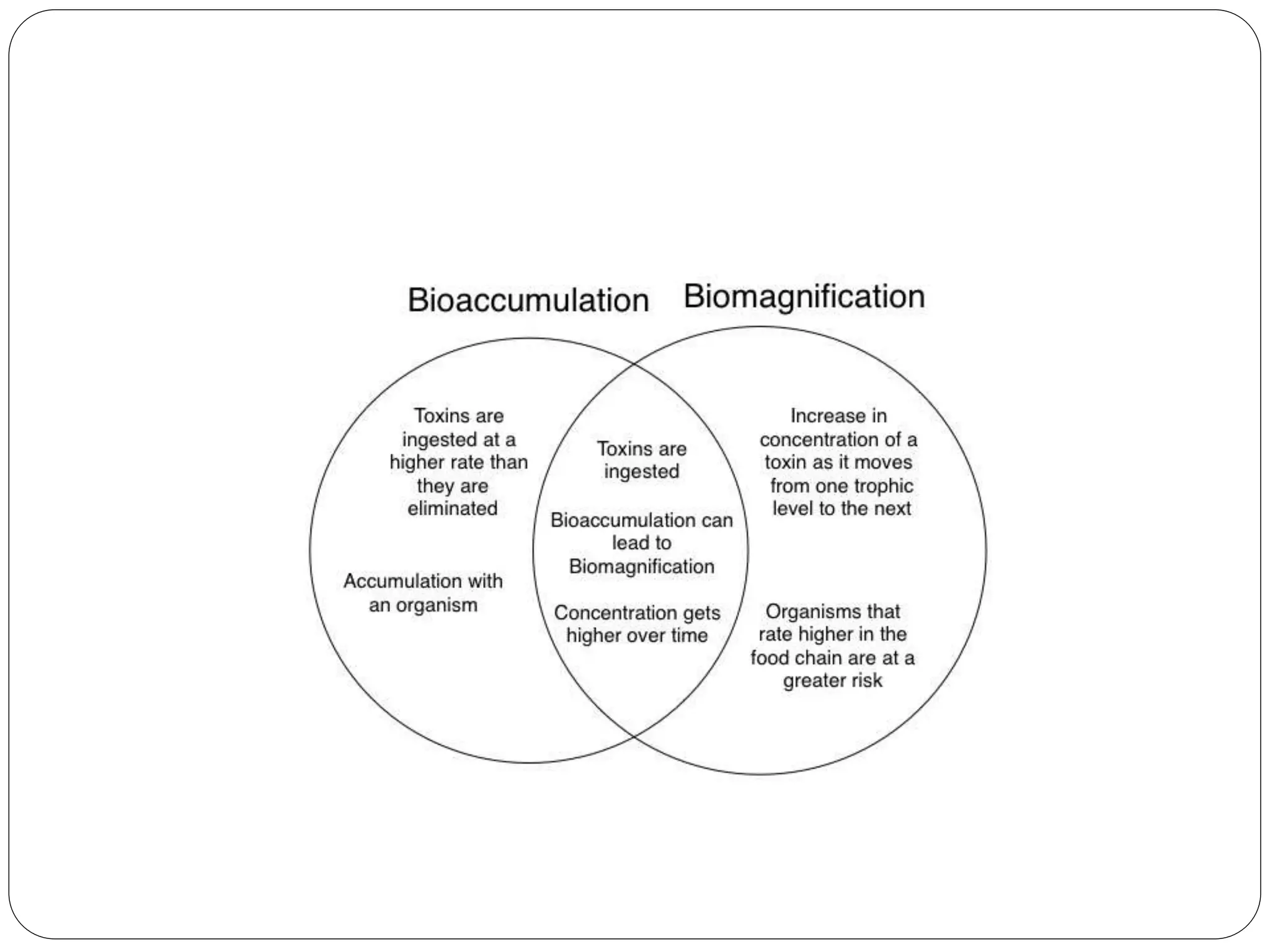
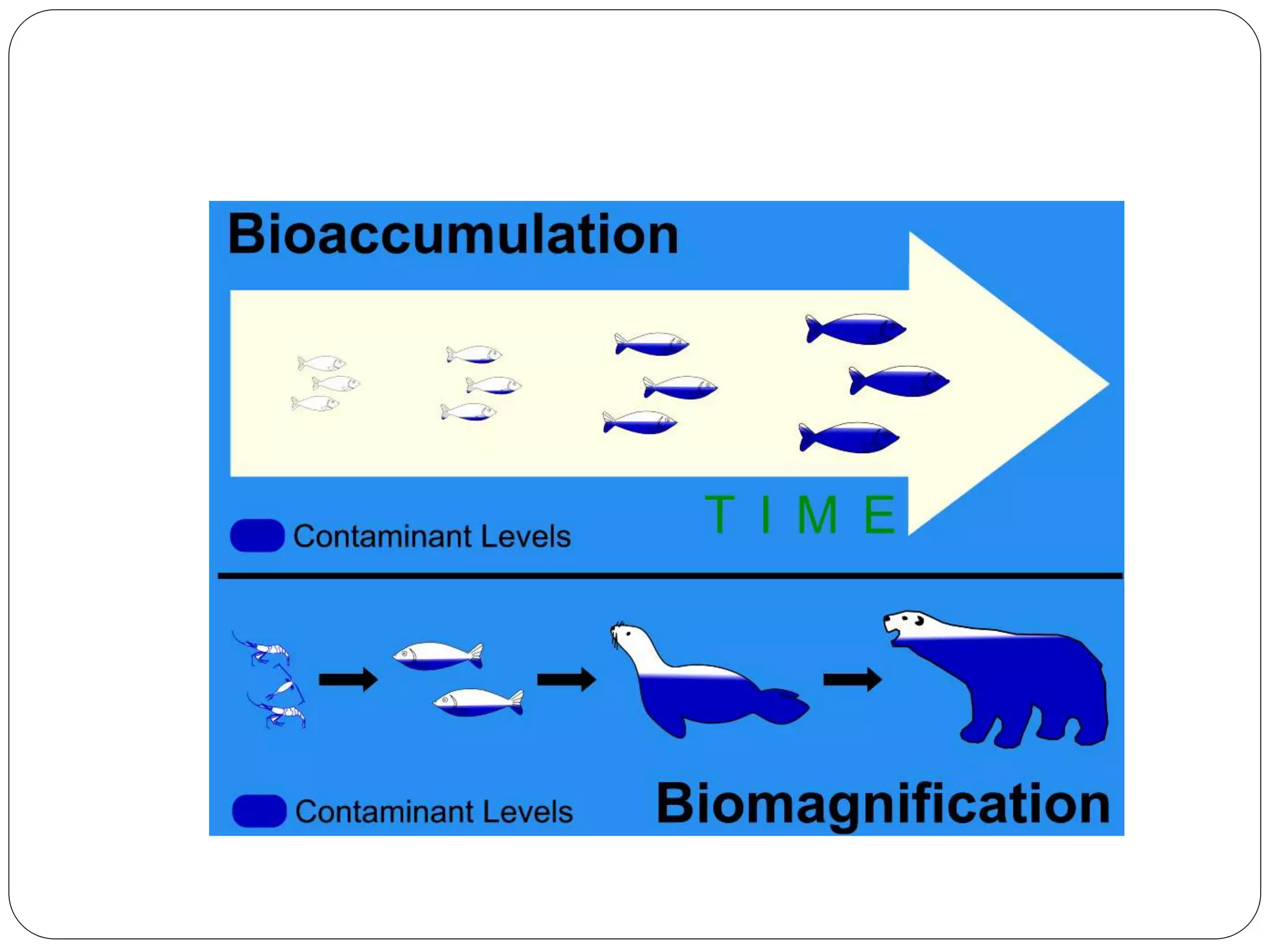
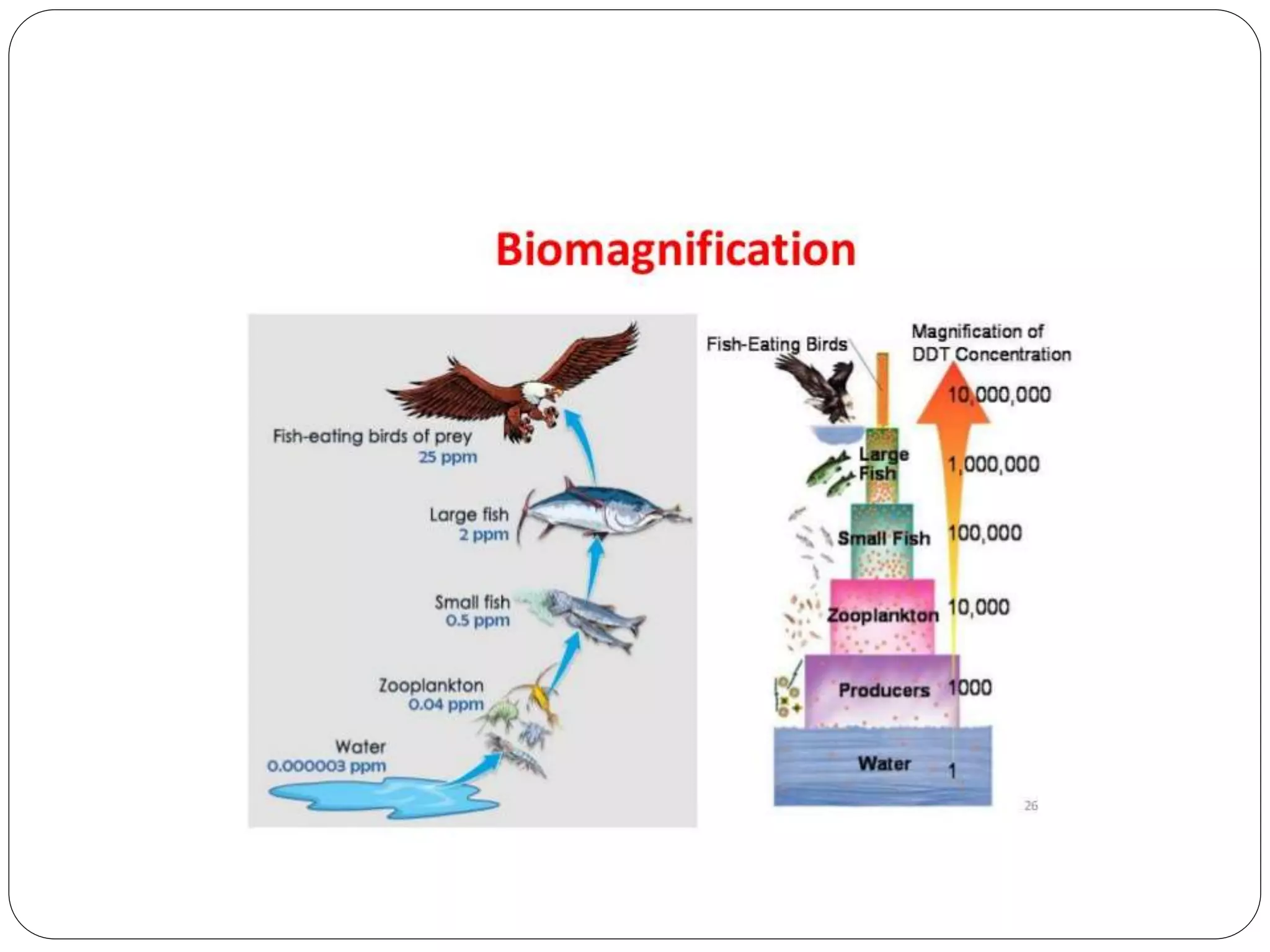
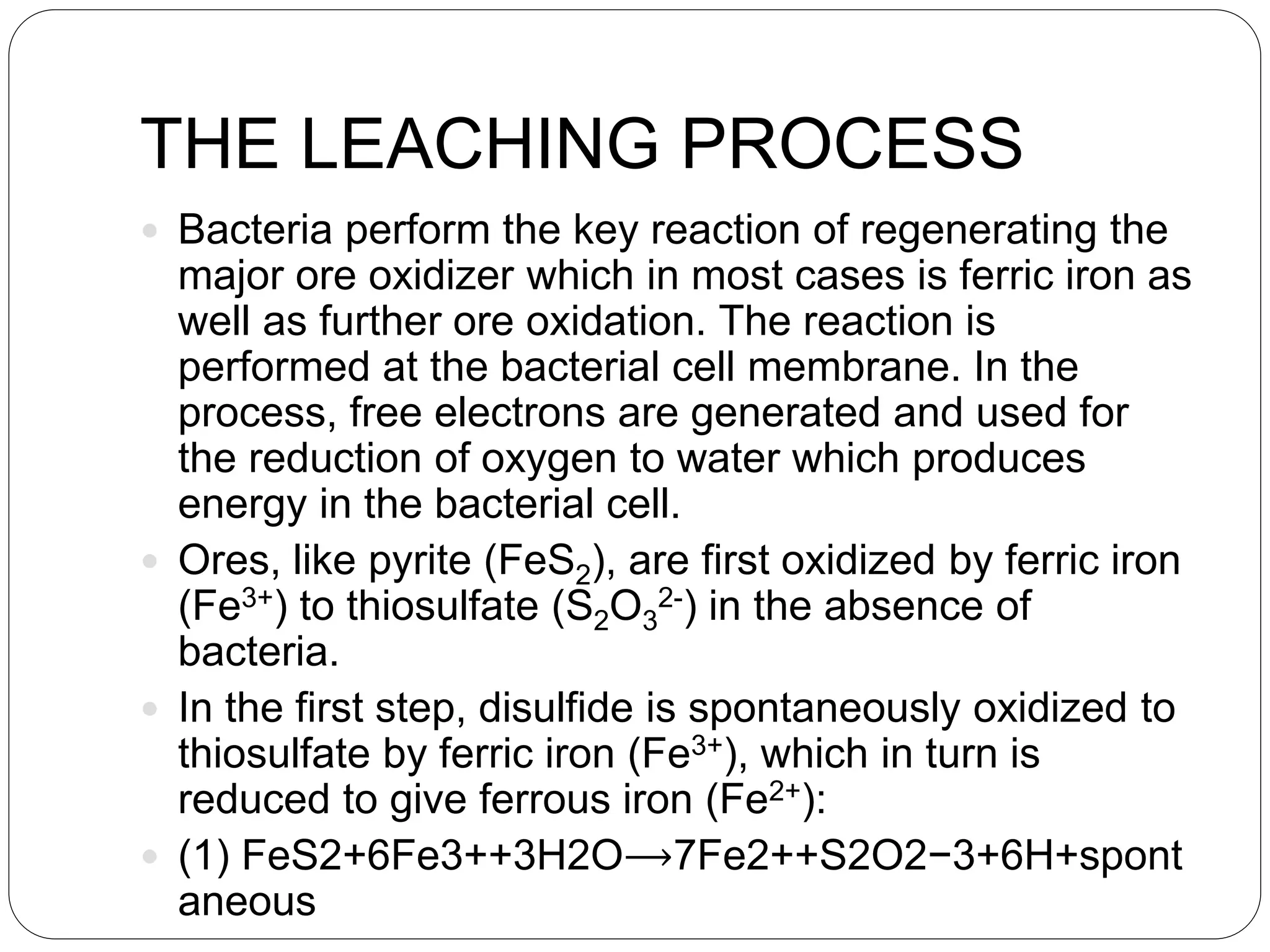
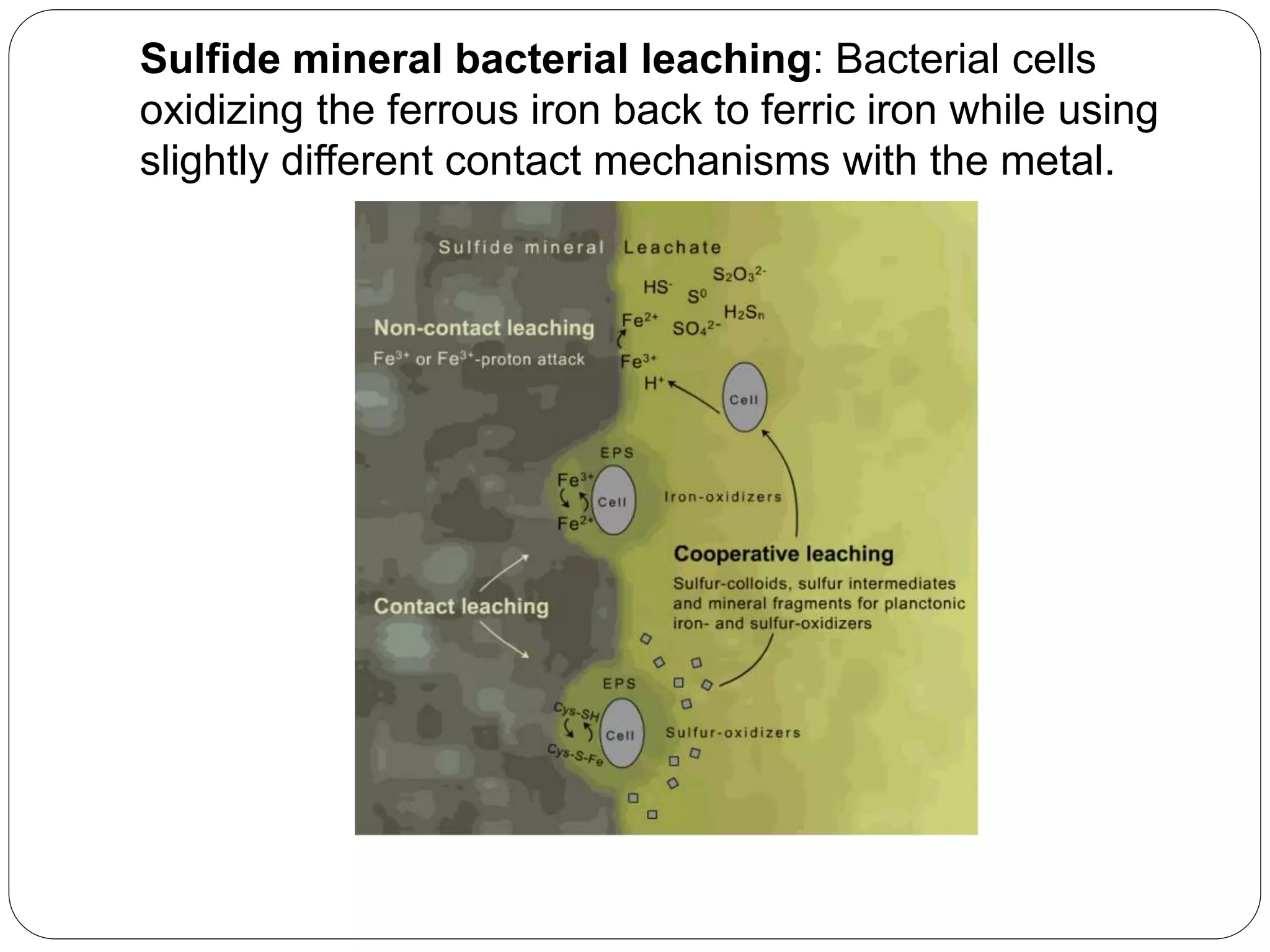
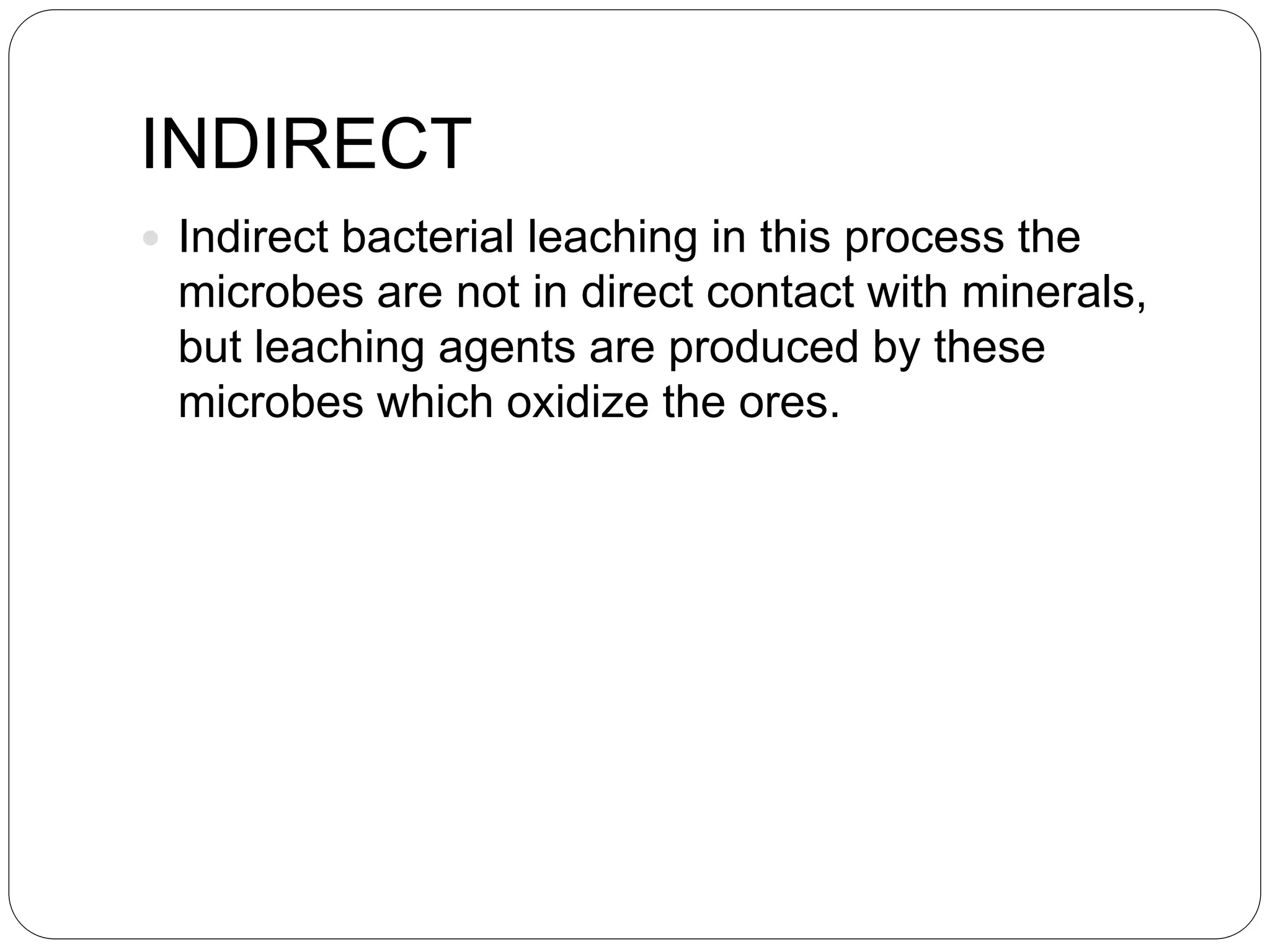
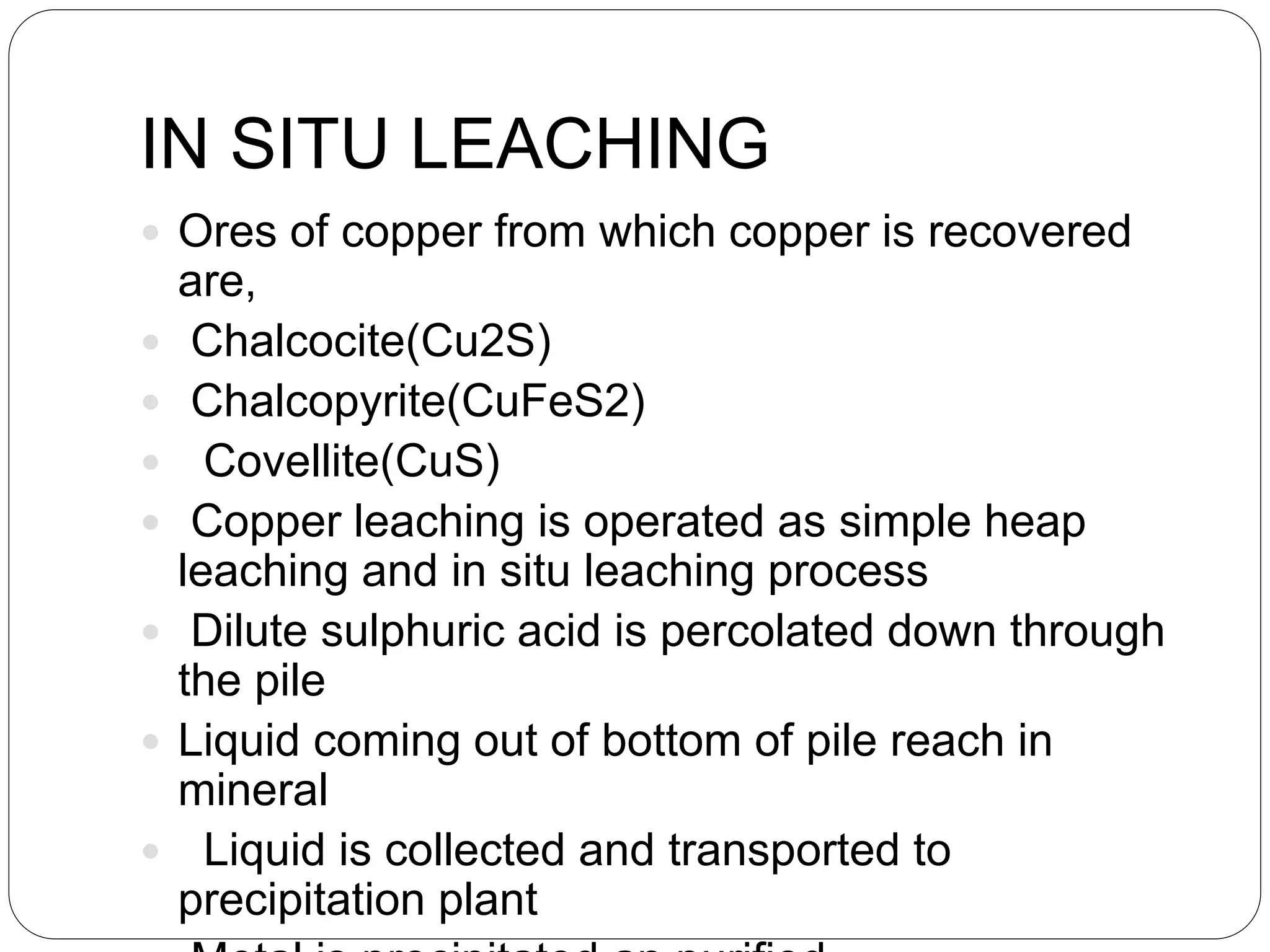
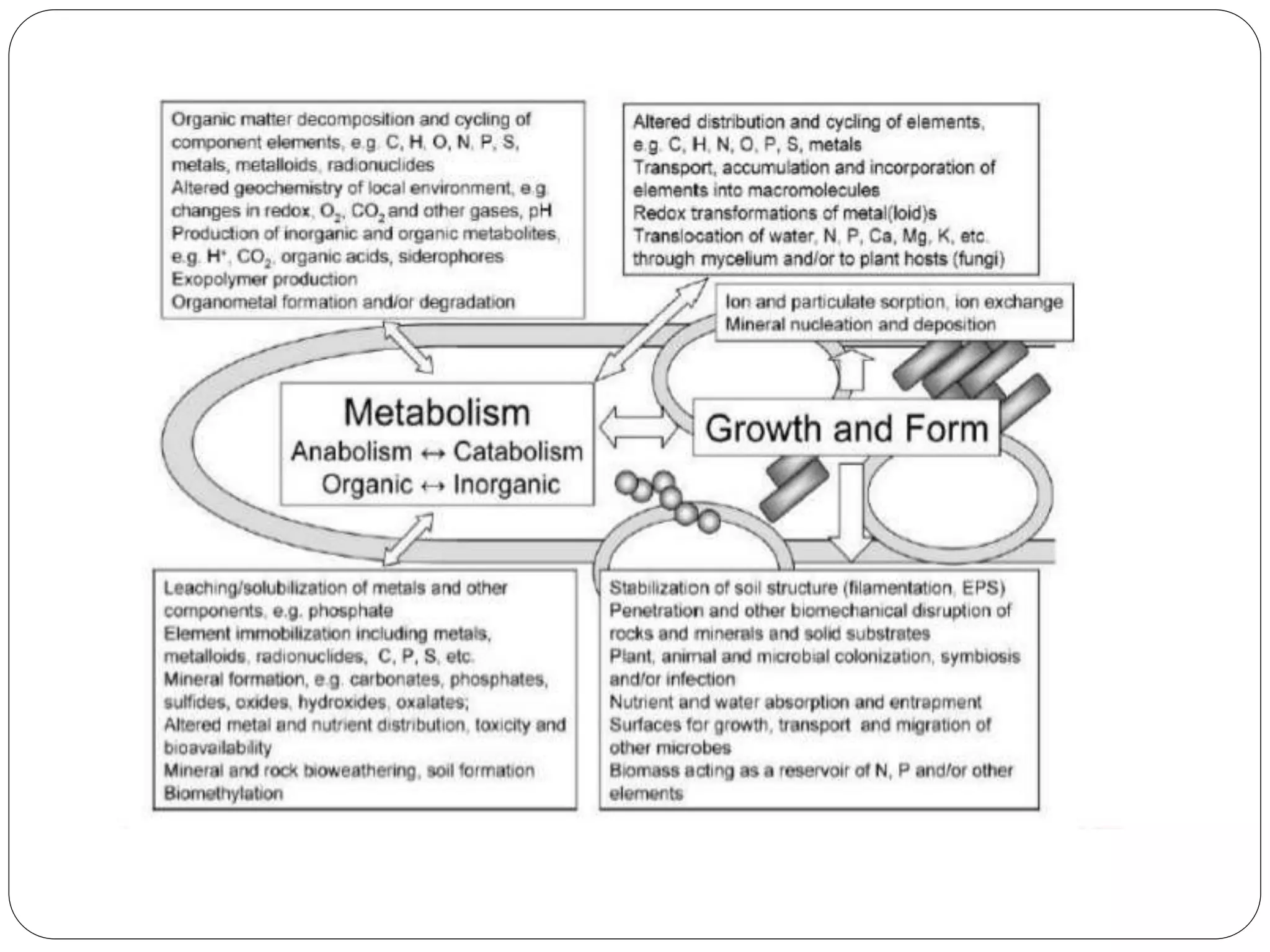
Microbial ore leaching, or bioleaching, utilizes microorganisms to extract metals from ores, offering a cost-effective and environmentally friendly alternative to traditional methods. Key bacteria, such as Acidithiobacillus ferrooxidans, facilitate reactions that lead to metal recovery while reducing pollution, although they may operate more slowly than chemical processes. The document further elaborates on various bioleaching processes, the role of fungi, and the significance of biomineralization and bioaccumulation in environmental contexts.













![ Uranium is extracted when insoluble tetravalent
uranium is oxidized with a hot H2So4/FeSo4
solution to make hexavalent uranium sulphate
pH required for the reaction is 1.5-3.5
Temperature: around 35 degree C following
reaction takes place, U2O+Fe2(SO4)3
UO2SO4+2FeSO4
Uranium leaching is an indirect process
When T.ferrooxidants are involved in uranium
extraction, they do not directly attack on ore but
on the iron oxidants.
The pyrite reaction is used for the initial
production of Fe Reaction; 2FeS+H2O+7 ½[O2]
Fe2[SO4]3+ H2SO4](https://image.slidesharecdn.com/newmicrosoftofficepowerpointpresentation-200915151023/75/ENRICHMENT-OF-ORES-BY-MICROORGANISMS-Bioaccumulation-and-biomineralization-19-2048.jpg)












![MERCURY POISONING
Alarming levels of toxic mercury were found in 264
samples of popular fish (like Rohu, Bhola, Tangra,
Aar, Bhetki and other fish varieties )collected across
West Bengal .[organisations :Toxics Link and DISHA
on 2012]
•The trend is applicable across the country
•While 52 cases had mercury concentrates in excess
of the Prevention of Food Adulteration (PFA) Act
standards of 0.5 ppm
• 129 of the fish showed methyl mercury levels (a
metabolized and more poisonous form of mercury)
exceeding the 0.25 ppm PFA stipulations. Mercury
levels in fish across West Bengal
Causes •coal firing •mining •thermal plants •industrial
effluents directly discharged into water bodies
•municipal waste water streams.](https://image.slidesharecdn.com/newmicrosoftofficepowerpointpresentation-200915151023/75/ENRICHMENT-OF-ORES-BY-MICROORGANISMS-Bioaccumulation-and-biomineralization-42-2048.jpg)