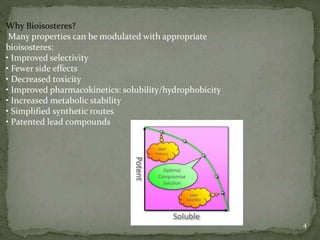
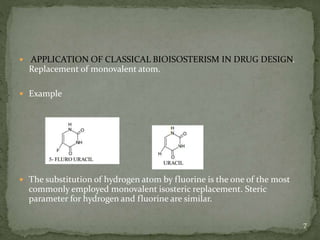
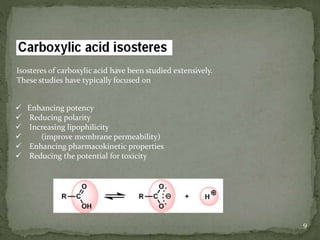
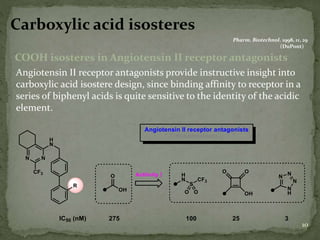
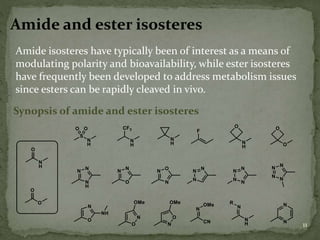
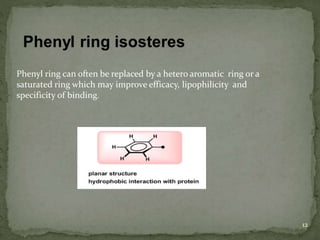
Bioisosterism is a strategy used in drug design that involves replacing one chemical group with another that has similar physical or chemical properties. This is done to improve properties like potency, selectivity, toxicity, and pharmacokinetics without significantly changing the chemical structure. Common bioisosteric replacements include replacing hydrogen with fluorine, replacing carboxylic acids with amides or esters, or replacing phenyl rings with heteroaromatic or saturated rings. The application of bioisosterism has been an important concept in medicinal chemistry for nearly 80 years and will continue to play a role in drug discovery and optimization.